Medical condition
| Cerebral venous sinus thrombosis | |
|---|---|
| Other names | Cerebral venous and sinus thrombosis, (superior) sagittal sinus thrombosis, dural sinus thrombosis, intracranial venous thrombosis, cerebral thrombophlebitis |
 | |
| Dural veins | |
| Specialty | Neurology |
| Treatment | Low molecular weight heparin |
Cerebral venous sinus thrombosis (CVST), cerebral venous and sinus thrombosis or cerebral venous thrombosis (CVT), is the presence of a blood clot in the dural venous sinuses (which drain blood from the brain), the cerebral veins, or both. Symptoms may include severe headache, visual symptoms, any of the symptoms of stroke such as weakness of the face and limbs on one side of the body, and seizures, which occur in around 40% of patients.
The diagnosis is usually by computed tomography (CT scan) or magnetic resonance imaging (MRI) to demonstrate obstruction of the venous sinuses. After confirmation of the diagnosis, investigations may be performed to determine the underlying cause, especially if one is not readily apparent.
Treatment is typically with anticoagulants (medications that suppress blood clotting) such as low molecular weight heparin. Rarely, thrombolysis (enzymatic destruction of the blood clot) or mechanical thrombectomy is used, although evidence for this therapy is limited. The disease may be complicated by raised intracranial pressure, which may warrant surgical intervention such as the placement of a shunt.
Signs and symptoms
Nine in ten people with cerebral venous thrombosis have a headache; this tends to worsen over the period of several days, but may also develop suddenly (thunderclap headache). The headache may be the only symptom. Many have symptoms of stroke: inability to move one or more limbs, weakness on one side of the face or difficulty speaking. The neurologic deficits related to central venous thromboses does not necessarily affect one side of the body or one arterial or brain territory as is more common "arterial" strokes. Bilateral 6th cranial nerve palsies may occur, causing abnormalities related to eye movement, but this is rare.
40% of people have seizures, although it is more common in women who develop sinus thrombosis peripartum (in the period before and after giving birth). These are mostly seizures affecting only one part of the body and unilateral (occurring on one side), but occasionally the seizures are generalised and rarely they lead to status epilepticus (persistent or recurrent seizure activity for a long period of time).
In the elderly, many of the aforementioned symptoms may not occur. Common symptoms in the elderly with this condition are otherwise unexplained changes in mental status and a depressed level of consciousness.
The pressure around the brain may rise, causing papilledema (swelling of the optic disc) which may be experienced as visual obscurations. In severely raised intracranial pressure, the level of consciousness is decreased, the blood pressure rises, the heart rate falls and there is abnormal posturing.
Focal neurologic deficits may occur hours to days after the headache in 50% of cases, this may present as hemiparesis (unilateral weakness) if due to infarction of the frontal or parietal lobe which are drained by the vein of Trolard. Focal deficits may also present as aphasia or confusion if the vein of Labbe (responsible for draining the temporal lobe) is affected.
Causes
Disorders that cause, or increase the risk for systemic venous thrombosis are associated with central venous thromboses. In children, head and neck infections and acute systemic illnesses are the primary cause of central venous thrombosis. Cerebral venous sinus thrombosis is more common in particular situations. 85% of people have at least one of these risk factors:
- Thrombophilia, a tendency to develop blood clots due to abnormalities in coagulation, e.g. factor V Leiden, deficiency of protein C, protein S or antithrombin, or related problems
- Nephrotic syndrome, a kidney problem causing protein loss in the urine
- Chronic inflammatory diseases, such as inflammatory bowel disease, lupus and Behçet's disease
- Pregnancy and puerperium (the period after giving birth)
- Particular blood disorders, especially polycythemia vera and paroxysmal nocturnal hemoglobinuria
- Use of estrogen-containing forms of hormonal contraception
- Meningitis and infections of the ear, nose and throat area such as mastoiditis and sinusitis
- Direct injury to the venous sinuses
- Medical procedures in the head and neck area
- Obesity, especially in combination with oral contraceptive use
- Sickle cell anemia
- Dehydration, primarily in infants and children
- Homocysteinemia
- Homocystinuria
Diagnosis
The diagnosis may be suspected on the basis of the symptoms, for example the combination of headache, signs of raised intracranial pressure and focal neurological abnormalities, or when alternative causes of headache and neurological abnormalities, such as a subarachnoid hemorrhage, have been excluded.
Imaging


There are various neuroimaging investigations that may detect cerebral sinus thrombosis. Cerebral edema and venous infarction may be apparent on any modality, but for the detection of the thrombus itself, the most commonly used tests are computed tomography (CT) and magnetic resonance imaging (MRI), both using various types of radiocontrast to perform a venogram and visualise the veins around the brain.
Computed tomography, with radiocontrast in the venous phase (CT venography or CTV), has a detection rate that in some regards exceeds that of MRI. The test involves injection into a vein (usually in the arm) of a radioopaque substance, and time is allowed for the bloodstream to carry it to the cerebral veins – at which point the scan is performed. It has a sensitivity of 75–100% (it detects 75–100% of all clots present), and a specificity of 81–100% (it would be incorrectly positive in 0–19%). In the first two weeks, the "empty delta sign" may be observed (in later stages, this sign may disappear). The empty delta sign is characterized by enhancement of the dural wall without intra-sinus enhancement.
Magnetic resonance venography employs the same principles, but uses MRI as a scanning modality. MRI has the advantage of being better at detecting damage to the brain itself as a result of the increased pressure on the obstructed veins, but it is not readily available in many hospitals and the interpretation may be difficult.
Cerebral angiography may demonstrate smaller clots than CT or MRI, and obstructed veins may give the "corkscrew appearance". This, however, requires puncture of the femoral artery with a sheath and advancing a thin tube through the blood vessels to the brain where radiocontrast is injected before X-ray images are obtained. It is therefore only performed if all other tests give unclear results or when other treatments may be administered during the same procedure.
D-dimer
There is an association between the D-dimer blood test and cerebral venous sinus thrombosis. This association however is not strong enough to rule out the diagnosis alone.
Further tests
In most cases, the direct cause for the cerebral sinus thrombosis is not readily apparent. Identifying a source of infection is crucial; it is common practice to screen for various forms of thrombophilia (a propensity to form blood clots).
Pathogenesis
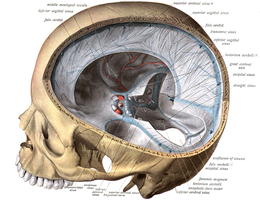
The veins of the brain, both the superficial veins and the deep venous system, empty into the dural venous sinuses, which carry blood back to the jugular vein and thence to the heart. In cerebral venous thrombosis, blood clots usually form both in the veins of the brain and the venous sinuses. The thrombosis of the veins themselves causes venous infarction (damage to brain tissue due to a congested and therefore insufficient blood supply). This results in cerebral edema (both vasogenic and cytotoxic edema), and leads to small petechial haemorrhages that may merge into large haematomas. Thrombosis of the sinuses is the main mechanism behind the increase in intracranial pressure due to decreased resorption of cerebrospinal fluid (CSF). The condition does not typically lead to hydrocephalus, however, because there is no difference in pressure between various parts of the brain. However, those who have deep cerebral venous sinus thrombosis or oedema at basal ganglia are more prone to hydrocephalus development.
Any blood clot forms due to an imbalance between coagulation (the formation of the insoluble blood protein fibrin) and fibrinolysis. The three major mechanisms for such an imbalance are enumerated in Virchow's triad: alterations in normal blood flow, injury to the blood vessel wall, and alterations in the constitution of blood (hypercoagulability). Most cases of cerebral venous sinus thrombosis are due to hypercoagulability. The inflammatory response and prolonged immobilization of patients with COVID-19 may also help explain the formation of CVST.
It is possible for the clot to break off and migrate (embolise) to the lungs, causing a pulmonary embolism. An analysis of earlier case reports concludes that this occurs in about 10% of cases, but has a very poor prognosis.
Central venous thromboses usually involve the dural sinuses with or without involvement of the cortical veins; isolated cortical venous thromboses are extremely rare with only about 100 cases reported.
Treatment
Various studies have investigated the use of anticoagulation to suppress blood clot formation in cerebral venous sinus thrombosis. Before these trials had been conducted, there had been a concern that small areas of hemorrhage in the brain would bleed further as a result of treatment; the studies showed that this concern was unfounded. Clinical practice guidelines now recommend heparin or low molecular weight heparin in the initial treatment, followed by warfarin, provided there are no other bleeding risks that would make these treatments unsuitable. Some experts discourage the use of anticoagulation if there is extensive hemorrhage; in that case, they recommend repeating the imaging after 7–10 days. If the hemorrhage has decreased in size, anticoagulants are started, while no anticoagulants are given if there is no reduction.
The duration of warfarin treatment depends on the circumstances and underlying causes of the condition. If the thrombosis developed under temporary circumstances (e.g. pregnancy), three months are regarded as sufficient. If the condition was unprovoked but there are no clear causes or a "mild" form of thrombophilia, 6 to 12 months is advised. If there is a severe underlying thrombosis disorder, warfarin treatment may need to continue indefinitely.
Heparin and platelet transfusions should not be used as a treatment for any form of cerebral venous thrombosis caused by immune thrombotic thrombocytopenias including Heparin induced thrombocytopenia (HIT), auto-immune heparin induced thrombocytopenia (aHIT) or vaccine induced immune thrombotic thrombocytopenia (VITT) due to unpredictable effects of heparin on anti-platelet factor-4 antibodies (PF-4). In cases of VITT, intravenous immune globulins (IVIG) are recommended as they block the anti-PF4 antibody interaction with platelets and a non-heparin anticoagulant. In refractory cases, plasma exchange may be used.
Thrombolysis (removal of the blood clot with "clot buster" medication) has been described, either systemically by injection into a vein or directly into the clot during angiography. The 2006 European Federation of Neurological Societies guideline recommends that thrombolysis is only used in people who deteriorate despite adequate treatment, and other causes of deterioration have been eliminated. It is unclear which drug and which mode of administration is the most effective. Bleeding into the brain and in other sites of the body is a major concern in the use of thrombolysis. American guidelines make no recommendation with regards to thrombolysis, stating that more research is needed.
In those where a venous infarct or hemorrhage causes significant compression of surrounding brain structures, decompressive craniectomy is sometimes required. Raised intracranial pressure, if severe or threatening vision, may require therapeutic lumbar puncture (removal of excessive cerebrospinal fluid), or neurosurgical treatment (optic nerve sheath fenestration or shunting). Venous stenting is emerging as a minimally invasive, safer alternative to shunting. In certain situations, anticonvulsants may be used to try to prevent seizures. These situations include focal neurological problems (e.g. inability to move a limb) and focal changes of the brain tissue on CT or MRI scan. Evidence to support or refute the use of antiepileptic drugs as a preventive measure, however, is lacking.
Prognosis
In 2004 the first adequately large scale study on the natural history and long-term prognosis of this condition was reported; this showed that at 16 months follow-up 57.1% of people had full recovery, 29.5%/2.9%/2.2% had respectively minor/moderate/severe symptoms or impairments, and 8.3% had died. Severe impairment or death were more likely in those aged over 37 years, male, affected by coma, mental status disorder, intracerebral hemorrhage, thrombosis of the deep cerebral venous system, central nervous system infection and cancer. A subsequent systematic review of nineteen studies in 2006 showed that mortality is about 5.6% during hospitalisation and 9.4% in total, while of the survivors 88% make a total or near-total recovery. After several months, two thirds of the cases has resolution ("recanalisation") of the clot. The rate of recurrence was low (2.8%).
In children with CVST the risk of death is high. Poor outcome is more likely if a child with CVST develops seizures or has evidence of venous infarction on imaging.
Epidemiology
Cerebral venous sinus thrombosis is rare, with an estimated 3-4 cases per million annual incidence in adults. While it may occur in all age groups, it is most common in the third decade. 75% are female. Given that older studies show no difference in incidence between men and women, it has been suggested that the use of oral contraceptives in women is behind the disparity between the sexes. A 1995 report from Saudi Arabia found a substantially larger incidence at 7 cases per 100,000; this was attributed to the fact that Behçet's disease, which increases risk of CVST, is more common in the Middle East.
A 1973 report found that CVST could be found on autopsy (examination of the body after death) in nine percent of all people. Many of these were elderly and had neurological symptoms in the period leading up to their death, and many developed concomitant heart failure. An estimated 0.3% incidence of CVST in patients infected with SARS-CoV-2.
In children, a Canadian study reported in 2001 that CVST occurs in 6.7 per million annually. 43% occur in the newborn (less than one month old), and a further 10% in the first year of life. Of the newborn, 84% were already ill, mostly from complications after childbirth and dehydration.
History
The first description of thrombosis of the cerebral veins and sinuses is attributed to the French physician Ribes, who in 1825 observed thrombosis of the sagittal sinus and cerebral veins in a man who had had seizures and delirium. Until the second half of the 20th century it remained a diagnosis generally made after death. In the 1940s, reports by Dr Charles Symonds and others allowed for the clinical diagnosis of cerebral venous thrombosis, using characteristic signs and symptoms and results of lumbar puncture.
Improvements on the diagnosis of cerebral venous sinus thrombosis in life were made with the introduction of venography in 1951, which also aided in the distinction from idiopathic intracranial hypertension, which has similar presenting signs and symptoms in many cases.
The British gynecologist Stansfield is credited with the introduction, in 1942, of the just recently introduced anticoagulant heparin in the treatment of CVST in 1942. Clinical trials in the 1990s finally resolved the concern about using anticoagulants in most cases of CVST.
COVID-19 vaccine
See also: Embolic and thrombotic events after COVID-19 vaccinationIn March 2021, the European Medicines Agency (EMA) announced that out of the around 20 million people who had received the Oxford–AstraZeneca COVID-19 vaccine, general blood clotting rates were normal, but that it had identified seven cases of disseminated intravascular coagulation, and eighteen cases of cerebral venous sinus thrombosis. It had been proposed that inadvertent injection of COVID-19 vaccine into deltoid muscle vasculature may result in vaccine distribution to distant tissues that may be causing these rare adverse reactions. A causal link with the vaccine, however, had not been proven, but the EMA decided to conduct further analysis and to inform recipients of the remote possibility of such rare syndromes. EMA confirmed that the vaccine's benefits still outweigh the risks and later released updated product information. and issued warnings to patients and healthcare professionals. The British Medicines and Healthcare products Regulatory Agency (MHRA) confirmed 79 cases of thrombosis, including 19 fatalities, within the first 20 million vaccinations in Great Britain. Guidelines on management of suspected cases was issued by the British Society for Haematology; it discouraged the use of heparin until it was clearer that heparin would not aggravate the thrombosis. On 13 April 2021 the Centers for Disease Control and Prevention paused the use of the Janssen COVID-19 vaccine in the United States due to six cases of CVST that occurred 6 to 13 days after administration. The recommended pause was lifted on 23 April 2021 following a safety review.
Notable cases
U.S. Secretary of State Hillary Clinton was hospitalized on 30 December 2012, for anticoagulation treatment of venous thrombosis of the right transverse sinus, which is located at the base of the brain. Clinton's thrombotic episode was discovered on an MRI scan done for follow-up of a cerebral concussion she had sustained 2.5 weeks previously, when she fell while suffering from gastroenteritis.
References
- ^ Al Rawahi B, Almegren M, Carrier M (September 2018). "The efficacy and safety of anticoagulation in cerebral vein thrombosis: A systematic review and meta-analysis". Thrombosis Research. 169: 135–139. doi:10.1016/j.thromres.2018.07.023. PMID 30056293. S2CID 51876550.
- Silvis S, Aguiar de Sousa D, Ferro J, Coutinho J (2017). "Cerebral venous thrombosis". Nat Rev Neurol. 13 (9): 555–565. doi:10.1038/nrneurol.2017.104. PMID 28820187. S2CID 54526187.
- ^ Stam J (2005). "Thrombosis of the cerebral veins and sinuses". N. Engl. J. Med. 352 (17): 1791–8. doi:10.1056/NEJMra042354. PMID 15858188. S2CID 42126852.
- Coutinho JM, Zuurbier SM, Bousser MG, Ji X, Canhão P, Roos YB, Crassard I, Nunes AP, Uyttenboogaart M, Chen J, Emmer BJ, Roosendaal SD, Houdart E, Reekers JA, van den Berg R, de Haan RJ, Majoie CB, Ferro JM, Stam J (1 August 2020). "Effect of Endovascular Treatment With Medical Management vs Standard Care on Severe Cerebral Venous Thrombosis: The TO-ACT Randomized Clinical Trial". JAMA Neurology. 77 (8): 966–973. doi:10.1001/jamaneurol.2020.1022. PMC 7235912. PMID 32421159.
- Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG (2005). "Headache as the only neurological sign of cerebral venous thrombosis: a series of 17 cases". J. Neurol. Neurosurg. Psychiatry. 76 (8): 1084–7. doi:10.1136/jnnp.2004.056275. PMC 1739763. PMID 16024884.
- ^ Ropper AH, Klein JP (1 July 2021). "Cerebral Venous Thrombosis". The New England Journal of Medicine. 385 (1): 59–64. doi:10.1056/NEJMra2106545. PMID 34192432. S2CID 235697487.
- ^ Einhäupl K, Bousser MG, de Bruijn SF, et al. (2006). "EFNS guideline on the treatment of cerebral venous and sinus thrombosis". Eur. J. Neurol. 13 (6): 553–9. doi:10.1111/j.1468-1331.2006.01398.x. PMID 16796579. S2CID 17618264.
- Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F (2005). "Cerebral vein and dural sinus thrombosis in elderly patients". Stroke. 36 (9): 1927–32. doi:10.1161/01.STR.0000177894.05495.54. PMID 16100024.
- Silvis S, Middeldorp S, Zuurbier S, Cannegieter S, Coutinho J (6 June 2016). "Risk Factors for Cerebral Venous Thrombosis". Seminars in Thrombosis and Hemostasis. 42 (6): 622–631. doi:10.1055/s-0036-1584132. PMID 27272966. S2CID 24465003.
- Zuurbier SM, Arnold M, Middeldorp S, Broeg-Morvay A, Silvis SM, Heldner MR, Meisterernst J, Nemeth B, Meulendijks ER, Stam J, Cannegieter SC, Coutinho JM (1 May 2016). "Risk of Cerebral Venous Thrombosis in Obese Women". JAMA Neurology. 73 (5): 579–584. doi:10.1001/jamaneurol.2016.0001. PMID 26974867.
- ^ Smith R, Hourihan MD (2007). "Investigating suspected cerebral venous thrombosis". The BMJ. 334 (7597): 794–5. doi:10.1136/bmj.39154.636968.47. PMC 1852020. PMID 17431266.
- ^ Smith E, Kumar V (June 2018). "BET 1: Does a normal D-dimer rule out cerebral venous sinus thrombosis (CVST)?". Emergency Medicine Journal. 35 (6): 396–397. doi:10.1136/emermed-2018-207777.1. PMID 29784833. S2CID 29151203.
- Zuurbier SM, van den Berg R, Troost D, Majoie CB, Stam J, Coutinho JM (2015). "Hydrocephalus in cerebral venous thrombosis". Journal of Neurology. 262 (4): 931–7. doi:10.1007/s00415-015-7652-4. PMC 4412517. PMID 25663408.
- ^ Gutiérrez-Zevallos JD, Guarniz-Huamán DA, Sánchez-Landers M (8 April 2021). "Cerebral venous thrombosis and COVID-19: A silent killer during the pandemic?". Revista de Neuro-Psiquiatria. 84 (1): 19–24. doi:10.20453/rnp.v84i1.3933.
- Diaz JM, Schiffman JS, Urban ES, Maccario M (1992). "Superior sagittal sinus thrombosis and pulmonary embolism: a syndrome rediscovered". Acta Neurol. Scand. 86 (4): 390–6. doi:10.1111/j.1600-0404.1992.tb05106.x. PMID 1455986. S2CID 29728372.
- Coutinho J, de Bruijn SF, Deveber G, Stam J (10 August 2011). "Anticoagulation for cerebral venous sinus thrombosis". Cochrane Database of Systematic Reviews. 2011 (8): CD002005. doi:10.1002/14651858.CD002005.pub2. ISSN 1469-493X. PMC 7065450. PMID 21833941.
- National Institute for Health and Clinical Excellence. Clinical guideline 68: Stroke. London, 2008.
- ^ Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA (July 2014). "Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack". Stroke. 45 (7): 2160–2236. doi:10.1161/STR.0000000000000024. PMID 24788967.
- Martinelli I, Franchini M, Mannucci PM (September 2008). "How I treat rare venous thromboses". Blood. 112 (13): 4818–23. doi:10.1182/blood-2008-07-165969. PMID 18805965.
- Avanali R, Gopalakrishnan MS, Devi BI, Bhat DI, Shukla DP, Shanbhag NC (15 May 2019). "Role of Decompressive Craniectomy in the Management of Cerebral Venous Sinus Thrombosis". Frontiers in Neurology. 10: 511. doi:10.3389/fneur.2019.00511. PMC 6529953. PMID 31156540.
- Li K, Ren M, Meng R, Ding Y, Rajah GB, Wang F, Ji X (2019). "Efficacy of stenting in patients with cerebral venous sinus thrombosis-related cerebral venous sinus stenosis". Journal of NeuroInterventional Surgery. 11 (3): 307–312. doi:10.1136/neurintsurg-2018-014328. ISSN 1759-8486. PMID 30389898. S2CID 53253904.
- Price M, Günther A, Kwan JS (21 April 2016). "Antiepileptic drugs for the primary and secondary prevention of seizures after intracranial venous thrombosis". Cochrane Database of Systematic Reviews. 2016 (4): CD005501. doi:10.1002/14651858.cd005501.pub4. hdl:10722/226344. PMC 7265129. PMID 27098266.
- Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F (2004). "Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT)". Stroke. 35 (3): 664–70. doi:10.1161/01.STR.0000117571.76197.26. PMID 14976332.
- Dentali F, Gianni M, Crowther MA, Ageno W (2006). "Natural history of cerebral vein thrombosis: a systematic review". Blood. 108 (4): 1129–34. doi:10.1182/blood-2005-12-4795. PMID 16609071.
- Jackson BF, Porcher FK, Zapton DT, Losek JD (September 2011). "Cerebral sinovenous thrombosis in children: diagnosis and treatment". Pediatr. Emerg. Care. 27 (9): 874–80, quiz 881–3. doi:10.1097/PEC.0b013e31822c9ccc. PMID 21926891. S2CID 11390572.
- ^ deVeber G, Andrew M, Adams C, et al. (August 2001). "Cerebral sinovenous thrombosis in children". N. Engl. J. Med. 345 (6): 417–23. doi:10.1056/NEJM200108093450604. PMID 11496852.
- Daif A, Awada A, al-Rajeh S, et al. (1 July 1995). "Cerebral venous thrombosis in adults. A study of 40 cases from Saudi Arabia". Stroke. 26 (7): 1193–5. doi:10.1161/01.str.26.7.1193. PMID 7604412.
- Towbin A (1 May 1973). "The syndrome of latent cerebral venous thrombosis: its frequency and relation to age and congestive heart failure". Stroke. 4 (3): 419–30. doi:10.1161/01.str.4.3.419. PMID 4713031.
- Ribes MF (1825). "Des recherches faites sur la phlebite". Rev. Med. Fr. Etrang. 3: 5–41.
- ^ Bousser MG, Chiras J, Bories J, Castaigne P (1 March 1985). "Cerebral venous thrombosis—a review of 38 cases". Stroke. 16 (2): 199–213. doi:10.1161/01.str.16.2.199. PMID 3975957.
- Symonds CP (September 1940). "Cerebral thrombophlebitis". Br. Med. J. 2 (4158): 348–52. doi:10.1136/bmj.2.4158.348. PMC 2179068. PMID 20783290.
- ^ Stansfield FR (April 1942). "Puerperal cerebral thrombophlebitis treated by heparin". Br. Med. J. 1 (4239): 436–438. doi:10.1136/bmj.1.4239.436. PMC 2164893. PMID 20784169.
- Ray BS, Dunbar HS, Dotter CT (January 1951). "Dural sinus venography as an aid to diagnosis in intracranial disease". J. Neurosurg. 8 (1): 23–37. doi:10.3171/jns.1951.8.1.0023. PMID 14804146.
- Ray BS, Dunbar HS (September 1951). "Thrombosis of the dural venous sinuses as a cause of pseudotumor cerebri". Ann. Surg. 134 (3): 376–86. doi:10.1097/00000658-195113430-00009. PMC 1802934. PMID 14869026.
- ^ "COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets". European Medicines Agency (EMA) (Press release). 18 March 2021. Retrieved 18 March 2021.
- Merchant H (1 July 2022). "Inadvertent injection of COVID-19 vaccine into deltoid muscle vasculature may result in vaccine distribution to distance tissues and consequent adverse reactions". Postgraduate Medical Journal. 98 (1161): e5. doi:10.1136/postgradmedj-2021-141119. ISSN 0032-5473. PMID 34588294. S2CID 238229239.
- "COVID-19 Vaccine AstraZeneca – Update on ongoing evaluation of blood clot cases". European Medicines Agency (EMA) (Press release). 25 March 2021. Retrieved 25 March 2021.
- "Annex 1: Summary of Product Characteristics" (PDF). European Medicines Agency (EMA). Retrieved 29 March 2021.
- "COVID-19 Vaccine AstraZeneca: Risk of thrombocytopenia and coagulation disorders". European Medicines Agency (EMA). 24 March 2021. Retrieved 25 March 2021.
- Merchant HA (24 March 2021). "CoViD vaccines and thrombotic events: EMA issued warning to patients and healthcare professionals". Journal of Pharmaceutical Policy and Practice. 14 (1): 32. doi:10.1186/s40545-021-00315-w. ISSN 2052-3211. PMC 7988638. PMID 33761987.
- "MHRA issues new advice, concluding a possible link between COVID-19 Vaccine AstraZeneca and extremely rare, unlikely to occur blood clots". 7 April 2021.
- "Cerebral Venous Sinus Thrombosis Incidence Is Higher Than Previously Thought". The British Government (gov.uk) (Press release). 7 April 2021. Retrieved 8 April 2021.
- Expert Haematology Panel (7 April 2021). "Guidance produced from the Expert Haematology Panel (EHP) focussed on Covid-19 Vaccine induced Thrombosis and Thrombocytopenia (VITT)" (PDF). BSH.org.uk. British Society for Haematology. Retrieved 29 April 2021.
- Marks P. "Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine". Retrieved 13 April 2021.
- "FDA and CDC Lift Recommended Pause on Johnson & Johnson (Janssen) COVID-19 Vaccine Use Following Thorough Safety Review". Retrieved 18 June 2021.
- Paul Richter. "Hillary Clinton expected to make full recovery from blood clot". Los Angeles Times. Retrieved 1 January 2013.
External links
| Classification | D |
|---|---|
| External resources |
- "Intracranial venous thrombosis – Patient UK". Archived from the original on 12 May 2008. Retrieved 28 October 2007.
- UCH Institute for Child Health. "Clinical guideline Cerebral Venous Sinus Thrombosis in Children". Archived from the original on 2 February 2009. Retrieved 28 October 2007.
| Cerebrovascular diseases including stroke | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischaemic stroke |
| ||||||||||||
| Haemorrhagic stroke |
| ||||||||||||
| Aneurysm | |||||||||||||
| Other | |||||||||||||
| Cardiovascular disease (vessels) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Arteries, arterioles and capillaries | |||||||||
| Veins |
| ||||||||
| Arteries or veins | |||||||||
| Blood pressure |
| ||||||||
Category: