| |
| Names | |
|---|---|
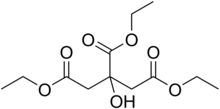
| Preferred IUPAC name Triethyl 2-hydroxypropane-1,2,3-tricarboxylate | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.974 |
| EC Number |
|
| E number | E1505 (additional chemicals) |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H20O7 |
| Molar mass | 276.283 g/mol |
| Appearance | Oily liquid |
| Density | 1.137 g/mL at 25 °C |
| Melting point | −55 °C (−67 °F; 218 K) |
| Boiling point | 294 °C (561 °F; 567 K) at 1 atm 235 °C at 150 mmHg |
| Solubility in water | 65 g/L |
| Magnetic susceptibility (χ) | −161.9·10 cm/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Triethyl citrate is an ester of citric acid. It is a colorless, odorless liquid used as a food additive, emulsifier and solvent (E number E1505) to stabilize foams, especially as whipping aid for egg white. It is also used in pharmaceutical coatings and plastics.
Triethyl citrate is also used as a plasticizer for polyvinyl chloride (PVC) and similar plastics.
Triethyl citrate has been used as a pseudo-emulsifier in e-cigarette juices. It functions essentially like lecithin used in food products, but with the possibility of vaporization which lecithin does not have.
References
- Triethyl citrate at Sigma-Aldrich
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 747. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Record of Triethyl citrate in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- "Triethyl citrate".
- William J. Stadelman; Owen J. Cotterill (1995). Egg Science and Technology. Haworth Press. ISBN 1-56022-855-5.
- Pharmaceutical Coatings Bulletin 102-4, morflex.com
- Hwan-Man Park; Manjusri Misra; Lawrence T. Drzal & Amar K. Mohanty (2004). ""Green" Nanocomposites from Cellulose Acetate Bioplastic and Clay: Effect of Eco-Friendly Triethyl Citrate Plasticizer". Biomacromolecules. 5 (6): 2281–2288. doi:10.1021/bm049690f. PMID 15530043.
- Erythropel, Hanno C; Anastas, Paul T; Krishnan-Sarin, Suchitra; O'Malley, Stephanie S; Jordt, Sven Eric; Zimmerman, Julie B (2020-04-27). "Differences in flavourant levels and synthetic coolant use between USA, EU and Canadian Juul products". Tobacco Control. 30 (4): tobaccocontrol–2019–055500. doi:10.1136/tobaccocontrol-2019-055500. ISSN 0964-4563. PMC 7606218. PMID 32341193.
This article about an ester is a stub. You can help Misplaced Pages by expanding it. |