Medical condition
| Esophageal cancer | |
|---|---|
| Other names | Oesophageal cancer |
 | |
| Endoscopic image of an esophageal adenocarcinoma | |
| Specialty | Gastroenterology General surgery oncology |
| Symptoms | Difficulty swallowing, weight loss, hoarse voice, enlarged lymph nodes around the collarbone, vomiting blood, blood in the stool |
| Types | Esophageal squamous-cell carcinoma, esophageal adenocarcinoma |
| Risk factors | Smoking tobacco, alcohol, very hot drinks, chewing betel nut, obesity, acid reflux |
| Diagnostic method | Tissue biopsy |
| Treatment | Surgery, chemotherapy, radiation therapy |
| Prognosis | Five-year survival rates ~15% |
| Frequency | 746,000 affected as of 2015 |
| Deaths | 509,000 (2018) |
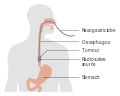
Esophageal cancer is cancer arising from the esophagus—the food pipe that runs between the throat and the stomach. Symptoms often include difficulty in swallowing and weight loss. Other symptoms may include pain when swallowing, a hoarse voice, enlarged lymph nodes ("glands") around the collarbone, a dry cough, and possibly coughing up or vomiting blood.
The two main sub-types of the disease are esophageal squamous-cell carcinoma (often abbreviated to ESCC), which is more common in the developing world, and esophageal adenocarcinoma (EAC), which is more common in the developed world. A number of less common types also occur. Squamous-cell carcinoma arises from the epithelial cells that line the esophagus. Adenocarcinoma arises from glandular cells present in the lower third of the esophagus, often where they have already transformed to intestinal cell type (a condition known as Barrett's esophagus).
Causes of the squamous-cell type include tobacco, alcohol, very hot drinks, poor diet, and chewing betel nut. The most common causes of the adenocarcinoma type are smoking tobacco, obesity, and acid reflux.
The disease is diagnosed by biopsy done by an endoscope (a fiberoptic camera). Prevention includes stopping smoking and eating a healthy diet. Treatment is based on the cancer's stage and location, together with the person's general condition and individual preferences. Small localized squamous-cell cancers may be treated with surgery alone with the hope of a cure. In most other cases, chemotherapy with or without radiation therapy is used along with surgery. Larger tumors may have their growth slowed with chemotherapy and radiation therapy. In the presence of extensive disease or if the affected person is not fit enough to undergo surgery, palliative care is often recommended.
As of 2018, esophageal cancer was the eighth-most common cancer globally with 572,000 new cases during the year. It caused about 509,000 deaths that year, up from 345,000 in 1990. Rates vary widely among countries, with about half of all cases occurring in China. It is around three times more common in men than in women. Outcomes are related to the extent of the disease and other medical conditions, but generally tend to be fairly poor, as diagnosis is often late. Five-year survival rates are around 13% to 18%.
Signs and symptoms
Prominent symptoms usually do not appear until the cancer has infiltrated over 60% of the circumference of the esophageal tube, by which time the tumor is already in an advanced stage. Onset of symptoms is usually caused by narrowing of the tube due to the physical presence of the tumor.
The first and the most common symptom is usually difficulty in swallowing, which is often experienced first with solid foods and later with softer foods and liquids. Pain when swallowing is less usual at first. Weight loss is often an initial sign in cases of squamous-cell carcinoma, though not usually in cases of adenocarcinoma. Eventual weight loss due to reduced appetite and undernutrition is common. Pain behind the breastbone or in the region around the stomach often feels like heartburn. The pain can frequently be severe, worsening when food of any sort is swallowed. Another sign may be an unusually husky, raspy, or hoarse-sounding cough, a result of the tumor affecting the recurrent laryngeal nerve.
The presence of the tumor may disrupt the normal contractions of the esophagus when swallowing. This can lead to nausea and vomiting, regurgitation of food and coughing. There is also an increased risk of aspiration pneumonia due to food entering the airways through the abnormal connections (fistulas) that may develop between the esophagus and the trachea (windpipe). Early signs of this serious complication may be coughing on drinking or eating. The tumor surface may be fragile and bleed, causing vomiting of blood. Compression of local structures occurs in advanced disease, leading to such problems as upper airway obstruction and superior vena cava syndrome. Hypercalcemia (excess calcium in the blood) may occur.
If the cancer has spread elsewhere, symptoms related to metastatic disease may appear. Common sites of spread include nearby lymph nodes, the liver, lungs and bone. Liver metastasis can cause jaundice and abdominal swelling (ascites). Lung metastasis can cause, among other symptoms, impaired breathing due to excess fluid around the lungs (pleural effusion), and dyspnea (the feelings often associated with impaired breathing).
Causes
The two main types (i.e. squamous-cell carcinoma and adenocarcinoma) have distinct sets of risk factors. Squamous-cell carcinoma is linked to lifestyle factors such as smoking and alcohol. Adenocarcinoma has been linked to effects of long-term acid reflux. Tobacco is a risk factor for both types. Both types are more common in people over 60 years of age.
Squamous-cell carcinoma
The two major risk factors for esophageal squamous-cell carcinoma are tobacco (smoking or chewing) and alcohol. The combination of tobacco and alcohol has a strong synergistic effect. Some data suggest that about half of all cases are due to tobacco and about one-third to alcohol, while over three-quarters of the cases in men are due to the combination of smoking and heavy drinking. Risks associated with alcohol appear to be linked to its aldehyde metabolite and to mutations in certain related enzymes. Such metabolic variants are relatively common in Asia.
Other relevant risk factors include regular consumption of very hot drinks (over 65 °C or 149 °F) and ingestion of caustic substances. High levels of dietary exposure to nitrosamines (chemical compounds found both in tobacco smoke and certain foodstuffs) also appear to be a relevant risk factor. Unfavorable dietary patterns seem to involve exposure to nitrosamines through processed and barbecued meats, pickled vegetables, etc., and a low intake of fresh foods. Other associated factors include nutritional deficiencies, low socioeconomic status, and poor oral hygiene. Chewing betel nut (areca) is an important risk factor in Asia.
Physical trauma may increase the risk. This may include the drinking of very hot drinks.
Adenocarcinoma
Male predominance is particularly strong in this type of esophageal cancer, which occurs about 7 to 10 times more frequently in men. This imbalance may be related to the characteristics and interactions of other known risk factors, including acid reflux and obesity.
GERD or Gastroesophageal reflux disease
The long-term erosive effects of acid reflux (an extremely common condition, also known as gastroesophageal reflux disease or GERD) have been strongly linked to this type of cancer. Longstanding GERD can induce a change of cell type in the lower portion of the esophagus in response to erosion of its squamous lining. This phenomenon, known as Barrett's esophagus, seems to appear about 20 years later in women than in men, possibly due to hormonal factors. At a mechanistic level, in the esophagus there is a small HOXA13 expressing compartment that is more resistant to bile and acids as the normal squamous epithelium and that is prone to both intestinal differentiation as well as oncogenic transformation. Following GERD this HOXA13-expressing compartment outcompetes the normal squamous compartment, leading to the intestinal aspect of the esophagus and increased propensity to the development of esophageal cancer. Having symptomatic GERD or bile reflux makes Barrett's esophagus more likely, which in turn raises the risk of further changes that can ultimately lead to adenocarcinoma. Bile reflux containing unconjugated bile acids, including deoxycholic acid and chenodeoxycholic acid, appears to contribute to esophageal adenocarcinoma carcinogenesis by inducing oxidative stress and DNA damage.The risk of developing adenocarcinoma in the presence of Barrett's esophagus is unclear, and may in the past have been overestimated.
Being obese or overweight both appear to be associated with increased risk. The association with obesity seems to be the strongest of any type of obesity-related cancer, though the reasons for this remain unclear. Abdominal obesity seems to be of particular relevance, given the closeness of its association with this type of cancer, as well as with both GERD and Barrett's esophagus. This type of obesity is characteristic of men. Physiologically, it stimulates GERD and also has other chronic inflammatory effects.
Helicobacter pylori infection (a common occurrence thought to have affected over half of the world's population) is not a risk factor for esophageal adenocarcinoma and actually appears to be protective. Despite being a cause of GERD and a risk factor for gastric cancer, the infection seems to be associated with a reduced risk of esophageal adenocarcinoma of as much as 50%. The biological explanation for a protective effect is somewhat unclear. One explanation is that some strains of H. pylori reduce stomach acid, thereby reducing damage by GERD. Decreasing rates of H. pylori infection in Western populations over recent decades, which have been linked to less overcrowding in households, could be a factor in the concurrent increase in esophageal adenocarcinoma.
Female hormones may also have a protective effect, as EAC is not only much less common in women but develops later in life, by an average of 20 years. Although studies of many reproductive factors have not produced a clear picture, risk seems to decline for the mother in line with prolonged periods of breastfeeding.
Tobacco smoking increases risk, but the effect in esophageal adenocarcinoma is slight compared to that in squamous cell carcinoma, and alcohol has not been demonstrated to be a cause.
Related conditions
- Head and neck cancer is associated with second primary tumors in the region, including esophageal squamous-cell carcinomas, due to field cancerization (i.e. a regional reaction to long-term carcinogenic exposure).
- History of radiation therapy for other conditions in the chest is a risk factor for esophageal adenocarcinoma.
- Corrosive injury to the esophagus by accidentally or intentionally swallowing caustic substances is a risk factor for squamous cell carcinoma.
- Tylosis with esophageal cancer is a rare familial disease with autosomal dominant inheritance that has been linked to a mutation in the RHBDF2 gene, present on chromosome 17: it involves thickening of the skin of the palms and soles and a high lifetime risk of squamous cell carcinoma.
- Achalasia (i.e. lack of the involuntary reflex in the esophagus after swallowing) appears to be a risk factor for both main types of esophageal cancer, at least in men, due to stagnation of trapped food and drink.
- Plummer–Vinson syndrome (a rare disease that involves esophageal webs) is also a risk factor.
- There is some evidence suggesting a possible causal association between human papillomavirus (HPV) and esophageal squamous-cell carcinoma. The relationship is unclear. Possible relevance of HPV could be greater in places that have a particularly high incidence of this form of the disease, as in some Asian countries, including China.
- There is an association between celiac disease and esophageal cancer. People with untreated celiac disease have a higher risk, but this risk decreases with time after diagnosis, probably due to the adoption of a gluten-free diet, which seems to have a protective role against development of malignancy in people with celiac disease. However, the delay in diagnosis and initiation of a gluten-free diet seems to increase the risk of malignancy. Moreover, in some cases the detection of celiac disease is due to the development of cancer, whose early symptoms are similar to some that may appear in celiac disease.
Diagnosis

Clinical evaluation
Although an occlusive tumor may be suspected on a barium swallow or barium meal, the diagnosis is best made with an examination using an endoscope. This involves the passing of a flexible tube with a light and camera down the esophagus and examining the wall, and is called an esophagogastroduodenoscopy. Biopsies taken of suspicious lesions are then examined histologically for signs of malignancy.
Additional testing is needed to assess how much the cancer has spread (see § Staging, below). Computed tomography (CT) of the chest, abdomen and pelvis can evaluate whether the cancer has spread to adjacent tissues or distant organs (especially liver and lymph nodes). The sensitivity of a CT scan is limited by its ability to detect masses (e.g. enlarged lymph nodes or involved organs) generally larger than 1 cm. Positron emission tomography is also used to estimate the extent of the disease and is regarded as more precise than CT alone. PET/MR as a novel modality has shown promising results in preoperative staging with fair feasibility and good correlation in comparison to PET/CT. It can enhance tissue differentiation with lowering the radiation dose to the patient. Esophageal endoscopic ultrasound can provide staging information regarding the level of tumor invasion, and possible spread to regional lymph nodes.
The location of the tumor is generally measured by the distance from the teeth. The esophagus (25 cm or 10 in long) is commonly divided into three parts for purposes of determining the location. Adenocarcinomas tend to occur nearer the stomach and squamous cell carcinomas nearer the throat, but either may arise anywhere in the esophagus.
-
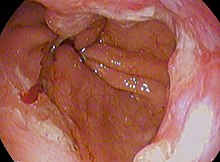
 Endoscopic image of Barrett esophagus – a frequent precursor of esophageal adenocarcinoma
Endoscopic image of Barrett esophagus – a frequent precursor of esophageal adenocarcinoma
-
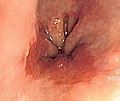
 Endoscopy and radial endoscopic ultrasound images of a submucosal tumor in the central portion of the esophagus
Endoscopy and radial endoscopic ultrasound images of a submucosal tumor in the central portion of the esophagus
-
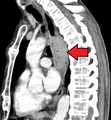
Contrast CT scan showing an esophageal tumor (axial view)
-
Contrast CT scan showing an esophageal tumor (coronal view)
-
 Esophageal cancer
Esophageal cancer
-
 Micrograph showing histopathological appearance of an esophageal adenocarcinoma (dark blue – upper-left of image) and normal squamous epithelium (upper-right of image) at H&E staining
Micrograph showing histopathological appearance of an esophageal adenocarcinoma (dark blue – upper-left of image) and normal squamous epithelium (upper-right of image) at H&E staining
Types
Esophageal cancers are typically carcinomas that arise from the epithelium, or surface lining, of the esophagus. Most esophageal cancers fall into one of two classes: esophageal squamous-cell carcinomas (ESCC), which are similar to head and neck cancer in their appearance and association with tobacco and alcohol consumption—and esophageal adenocarcinomas (EAC), which are often associated with a history of GERD and Barrett's esophagus. A rule of thumb is that a cancer in the upper two-thirds is likely to be ESCC and one in the lower one-third EAC.
Rare histologic types of esophageal cancer include different variants of squamous-cell carcinoma, and non-epithelial tumors, such as leiomyosarcoma, malignant melanoma, rhabdomyosarcoma and lymphoma, among others.
Staging
Staging is based on the TNM staging system, which classifies the amount of tumor invasion (T), involvement of lymph nodes (N), and distant metastasis (M). The currently preferred classification is the 2010 AJCC staging system for cancer of the esophagus and the esophagogastric junction. To help guide clinical decision making, this system also incorporates information on cell type (ESCC, EAC, etc.), grade (degree of differentiation – an indication of the biological aggressiveness of the cancer cells), and tumor location (upper, middle, lower, or junctional).
-
 T1, T2, and T3 stages of esophageal cancer
T1, T2, and T3 stages of esophageal cancer
-
 Stage T4 esophageal cancer
Stage T4 esophageal cancer
-
 Esophageal cancer with spread to lymph nodes
Esophageal cancer with spread to lymph nodes
Prevention
Prevention includes stopping smoking or chewing tobacco. Overcoming addiction to areca chewing in Asia is another promising strategy for the prevention of esophageal squamous-cell carcinoma. The risk can also be reduced by maintaining a normal body weight. According to a 2022 umbrella review, calcium intake could be associated with lower risk.
According to the National Cancer Institute, "diets high in cruciferous (cabbage, broccoli/broccolini, cauliflower, Brussels sprouts) and green and yellow vegetables and fruits are associated with a decreased risk of esophageal cancer." Dietary fiber is thought to be protective, especially against esophageal adenocarcinoma. There is no evidence that vitamin supplements change the risk.
Screening
People with Barrett's esophagus (a change in the cells lining the lower esophagus) are at much higher risk, and may receive regular endoscopic screening for the early signs of cancer. Because the benefit of screening for adenocarcinoma in people without symptoms is unclear, it is not recommended in the United States. Some areas of the world with high rates of squamous-carcinoma have screening programs.
Management



Treatment is best managed by a multidisciplinary team covering the various specialties involved. Adequate nutrition must be assured, and appropriate dental care is essential. Factors that influence treatment decisions include the stage and cellular type of cancer (EAC, ESCC, and other types), along with the person's general condition and any other diseases that are present.
In general, treatment with a curative intention is restricted to localized disease, without distant metastasis: in such cases a combined approach that includes surgery may be considered. Disease that is widespread, metastatic or recurrent is managed palliatively: in this case, chemotherapy may be used to lengthen survival, while treatments such as radiotherapy or stenting may be used to relieve symptoms and make it easier to swallow.
Surgery
Further information: EsophagectomyIf the cancer has been diagnosed while still in an early stage, surgical treatment with a curative intention may be possible. Some small tumors that only involve the mucosa or lining of the esophagus may be removed by endoscopic mucosal resection (EMR). Otherwise, curative surgery of early-stage lesions may entail removal of all or part of the esophagus (esophagectomy), although this is a difficult operation with a relatively high risk of mortality or post-operative difficulties. The benefits of surgery are less clear in early-stage ESCC than EAC. There are a number of surgical options, and the best choices for particular situations remain the subject of research and discussion. As well as characteristics and location of the tumor, other factors include the patient's condition, and the type of operation with which the surgical team is most experienced.
The likely quality of life after treatment is a relevant factor when considering surgery. Surgical outcomes are likely better in large centers where the procedures are frequently performed. If the cancer has spread to other parts of the body, esophagectomy is nowadays not normally performed.
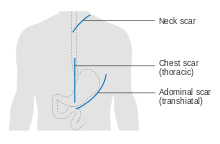
Esophagectomy is the removal of a segment of the esophagus; as this shortens the length of the remaining esophagus, some other segment of the digestive tract is pulled up through the chest cavity and interposed. This is usually the stomach or part of the large intestine (colon) or jejunum. Reconnection of the stomach to a shortened esophagus is called an esophagogastric anastomosis.
Esophagectomy can be performed using several methods. The choice of the surgical approach depends on the characteristics and location of the tumor, and the preference of the surgeon. Clear evidence from clinical trials for which approaches give the best outcomes in different circumstances is lacking. A first decision, regarding the point of entry, is between a transhiatial and a transthoracic procedure. The more recent transhiatial approach avoids the need to open the chest; instead the surgeon enters the body through an incision in the lower abdomen and another in the neck. The lower part of the esophagus is freed from the surrounding tissues and cut away as necessary. The stomach is then pushed through the esophageal hiatus (the hole where the esophagus passes through the diaphragm) and is joined to the remaining upper part of the esophagus at the neck.
The traditional transthoracic approach enters the body through the chest, and has a number of variations. The thoracoabdominal approach opens the abdominal and thoracic cavities together, the two-stage Ivor Lewis (also called Lewis–Tanner) approach involves an initial laparotomy and construction of a gastric tube, followed by a right thoracotomy to excise the tumor and create an esophagogastric anastomosis. The three-stage McKeown approach adds a third incision in the neck to complete the cervical anastomosis. Recent approaches by some surgeons use what is called extended esophagectomy, where more surrounding tissue, including lymph nodes, is removed en bloc.
If the person cannot swallow at all, an esophageal stent may be inserted to keep the esophagus open; stents may also assist in occluding fistulas. A nasogastric tube may be necessary to continue feeding while treatment for the tumor is given, and some patients require a gastrostomy (feeding hole in the skin that gives direct access to the stomach). The latter two are especially important if the patient tends to aspirate food or saliva into the airways, predisposing for aspiration pneumonia.
Chemotherapy and radiotherapy
Chemotherapy depends on the tumor type, but tends to be cisplatin-based (or carboplatin or oxaliplatin) every three weeks with fluorouracil (5-FU) either continuously or every three weeks. In more recent studies, addition of epirubicin was better than other comparable regimens in advanced nonresectable cancer. Chemotherapy may be given after surgery (adjuvant, i.e. to reduce risk of recurrence), before surgery (neoadjuvant) or if surgery is not possible; in this case, cisplatin and 5-FU are used. Ongoing trials compare various combinations of chemotherapy; the phase II/III REAL-2 trial – for example – compares four regimens containing epirubicin and either cisplatin or oxaliplatin, and either continuously infused fluorouracil or capecitabine.
Radiotherapy is given before, during, or after chemotherapy or surgery, and sometimes on its own to control symptoms. In patients with localised disease but contraindications to surgery, "radical radiotherapy" may be used with curative intent.
Other approaches
Forms of endoscopic therapy have been used for stage 0 and I disease: endoscopic mucosal resection (EMR) and mucosal ablation using radiofrequency ablation, photodynamic therapy, Nd-YAG laser, or argon plasma coagulation.
Laser therapy is the use of high-intensity light to destroy tumor cells while affecting only the treated area. This is typically done if the cancer cannot be removed by surgery. The relief of a blockage can help with pain and difficulty swallowing. Photodynamic therapy, a type of laser therapy, involves the use of drugs that are absorbed by cancer cells; when exposed to a special light, the drugs become active and destroy the cancer cells.
-
 Internal radiotherapy for esophageal cancer
Internal radiotherapy for esophageal cancer
-
 Self-expandable metallic stents are sometimes used for palliative care
Self-expandable metallic stents are sometimes used for palliative care
Follow-up
Patients are followed closely after a treatment regimen has been completed. Frequently, other treatments are used to improve symptoms and maximize nutrition.
Prognosis
In general, the prognosis of esophageal cancer is quite poor, because most patients present with advanced disease. By the time the first symptoms (such as difficulty swallowing) appear, the disease has already progressed. The overall five-year survival rate (5YSR) in the United States is around 15%, with most people dying within the first year of diagnosis. The latest survival data for England and Wales (patients diagnosed during 2007) show that only one in ten people survives esophageal cancer for at least ten years.
Individualized prognosis depends largely on stage. Those with cancer restricted entirely to the esophageal mucosa have about an 80% 5YSR, but submucosal involvement brings this down to less than 50%. Extension into the muscularis propria (muscle layer of the esophagus) suggests a 20% 5YSR, and extension to the structures adjacent to the esophagus predict a 7% 5YSR. Patients with distant metastases (who are not candidates for curative surgery) have a less than 3% 5YSR.
Epidemiology


Esophageal cancer is the eighth-most frequently-diagnosed cancer worldwide, and because of its poor prognosis, it is the sixth most-common cause of cancer-related deaths. It caused about 400,000 deaths in 2012, accounting for about 5% of all cancer deaths (about 456,000 new cases were diagnosed, representing about 3% of all cancers).
ESCC (esophageal squamous-cell carcinoma) comprises 60–70% of all cases of esophageal cancer worldwide, while EAC (esophageal adenocarcinoma) accounts for a further 20–30% (melanomas, leiomyosarcomas, carcinoids and lymphomas are less common types). The incidence of the two main types of esophageal cancer varies greatly between different geographical areas. In general, ESCC is more common in the developing world, and EAC is more common in the developed world.
The worldwide incidence rate of ESCC in 2012 was 5.2 new cases per 100,000 person-years, with a male predominance (7.7 per 100,000 in men vs. 2.8 in women). It was the common type in 90% of the countries studied. ESCC is particularly frequent in the so-called "Asian esophageal cancer belt", an area that passes through northern China, southern Russia, north-eastern Iran, northern Afghanistan and eastern Turkey. In 2012, about 80% of ESCC cases worldwide occurred in central and south-eastern Asia, and over half (53%) of all cases were in China. The countries with the highest estimated national incidence rates were (in Asia) Mongolia and Turkmenistan and (in Africa) Malawi, Kenya and Uganda. The problem of esophageal cancer has long been recognized in the eastern and southern parts of Sub-Saharan Africa, where ESCC appears to predominate.
In Western countries, EAC has become the dominant form of the disease, following an increase in incidence over recent decades (in contrast to the incidence of ESCC, which has remained largely stable). In 2012, the global incidence rate for EAC was 0.7 per 100,000 with a strong male predominance (1.1 per 100,000 in men vs. 0.3 in women). Areas with particularly high incidence rates include northern and western Europe, North America and Oceania. The countries with highest recorded rates were the UK, Netherlands, Ireland, Iceland and New Zealand.
United States
In the United States, esophageal cancer is the seventh-leading cause of cancer-related deaths among males (making up 4% of the total). The National Cancer Institute estimated that there were about 18,000 new cases and more than 15,000 deaths from esophageal cancer in 2013; the American Cancer Society estimated that during 2014, about 18,170 new esophageal cancer cases would be diagnosed, resulting in 15,450 deaths.
The squamous-cell carcinoma type is more common among African American males with a history of heavy smoking or alcohol use. Until the 1970s, squamous-cell carcinoma accounted for the vast majority of esophageal cancers in the United States. In recent decades, incidence of adenocarcinoma of the esophagus (which is associated with Barrett's esophagus) steadily rose in the United States to the point that it has now surpassed squamous-cell carcinoma. In contrast to squamous-cell carcinoma, esophageal adenocarcinoma is more common in white American men (over the age of 60) than it is in African Americans. Multiple reports indicate esophageal adenocarcinoma incidence has increased during the past 20 years, especially in non-Hispanic white men. Esophageal adenocarcinoma age-adjusted incidence increased in New Mexico from 1973 to 2002. This increase was found in non-Hispanic whites and Hispanics and became predominant in non-Hispanic whites. Esophageal cancer incidence and mortality rates for African Americans continue to be higher than the rate for Causasians. However, incidence and mortality of esophageal cancer has significantly decreased among African Americans since the early 1980s, whereas with whites it has continued to increase. Between 1975 and 2004, incidence of the adenocarcinoma type increased among white American males by over 460% and among white American females by 335%.
United Kingdom
The incidence of esophageal adenocarcinoma has risen considerably in the UK in recent decades. Overall, esophageal cancer is the thirteenth most common cancer in the UK (around 8,300 people were diagnosed with the disease in 2011), and it is the sixth most common cause of cancer death (around 7,700 people died in 2012).
Society and culture
Notable cases
See also: Category:Deaths from esophageal cancerHumphrey Bogart, actor, died of esophageal cancer in 1957, aged 57.
Billy Strayhorn, American jazz composer, pianist, lyricist, and arranger, who collaborated with bandleader and composer Duke Ellington, died of esophageal cancer in 1967 at age 51.
Actor John Thaw died of esophageal cancer in 2002, at the age of 60.
Christopher Hitchens, author and journalist, died of esophageal cancer in 2011, aged 62.
Morrissey in October 2015 stated he has the disease and has described his experience when he first heard he had it.
Mako Iwamatsu, voice actor for Avatar: The Last Airbender as General Iroh and Samurai Jack as Aku, died of esophageal cancer in 2006, aged 72.
Robert Kardashian, attorney and businessman, died of esophageal cancer in 2003, aged 59.
Traci Braxton, singer and reality TV star, died of esophageal cancer in 2022, aged 50.
Andrew Bonar Law resigned as Prime Minister of the United Kingdom in 1923 and died of throat cancer shortly after aged 65.
Ed Sullivan, host of the prominent self-titled television program The Ed Sullivan Show, died of esophageal cancer in 1974 at the age of 73.
Lynn Yamada Davis, chef YouTube star, died of esophageal cancer in 2024, aged 67.
Research directions
The risk of esophageal squamous-cell carcinoma may be reduced in people using aspirin or related NSAIDs, but in the absence of randomized controlled trials the current evidence is inconclusive.
The genomics of esophageal adenocarcinoma is being studied using cancer genome sequencing. Esophageal adenocarcinoma is characterized by complex tumor genomes with heterogeneity within the tumor micro-environment.
See also
References
- ^ Ferri FF, ed. (2012). "Tumors". Ferri's clinical advisor 2013. Philadelphia, PA: Mosby (Elsevier). pp. 389–391. ISBN 978-0-323-08373-7. Archived from the original on 2015-09-19.
- ^ Montgomery EA, Basman FT, Brennan P, Malekzadeh R (2014). "Oesophageal Cancer". In Stewart BW, Wild CP (eds.). World Cancer Report 2014. World Health Organization. pp. 528–543. ISBN 978-92-832-0429-9.
- ^ Zhang HZ, Jin GF, Shen HB (June 2012). "Epidemiologic differences in esophageal cancer between Asian and Western populations". Chinese Journal of Cancer. 31 (6): 281–286. doi:10.5732/cjc.011.10390. PMC 3777490. PMID 22507220.
- ^ Akhtar S (February 2013). "Areca nut chewing and esophageal squamous-cell carcinoma risk in Asians: a meta-analysis of case-control studies". Cancer Causes & Control. 24 (2): 257–265. doi:10.1007/s10552-012-0113-9. PMID 23224324. S2CID 14356684.
- ^ Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D (October 2013). "Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology. 24 (Suppl 6): vi51–vi56. doi:10.1093/annonc/mdt342. PMID 24078662.
- ^ "SEER Stat Fact Sheets: Esophageal Cancer". National Cancer Institute. Archived from the original on 6 July 2014. Retrieved 18 June 2014.
- Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators) (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
- ^ "Esophageal Cancer Factsheet" (PDF). Global Cancer Observatory. Retrieved 8 November 2019.
- Even by those using the British English spelling "oesophagus"
- Kelsen D (2007). Gastrointestinal oncology: principles and practices (2nd ed.). Philadelphia, Pa.: Lippincott Williams & Wilkins. p. 4. ISBN 978-0-7817-7617-2. Archived from the original on 2015-09-25.
- Schottenfeld D, Fraumeni J, eds. (2006). Cancer epidemiology and prevention (3rd ed.). Oxford: Oxford University Press. p. 697. ISBN 978-0-19-974797-9. Archived from the original on 2015-10-31.
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–2128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMC 10790329. PMID 23245604. S2CID 1541253.
- ^ Enzinger PC, Mayer RJ (December 2003). "Esophageal cancer" (PDF). The New England Journal of Medicine. 349 (23): 2241–2252. doi:10.1056/NEJMra035010. PMID 14657432. Archived from the original (PDF) on 2014-07-14.
- ^ Mayer RJ (2008). "Gastrointestinal Tract Cancer". In Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J (eds.). Harrison's principles of internal medicine. Vol. 1 (18th ed.). New York: McGraw-Hill Medical Publishing Division. pp. 764–5. ISBN 978-0-07-174889-6.
- Cheifetz AS, Brown A, Curry M, Moss AC (2011). Oxford American Handbook of Gastroenterology and Hepatology. Oxford University Press. p. 106. ISBN 978-0-19-983012-1.
- ^ Pennathur A, Gibson MK, Jobe BA, Luketich JD (February 2013). "Oesophageal carcinoma". Lancet. 381 (9864): 400–412. doi:10.1016/S0140-6736(12)60643-6. PMID 23374478. S2CID 13550805.
- Yamada T (2011). Textbook of Gastroenterology. John Wiley & Sons. pp. 1590–1. ISBN 978-1-4443-5941-1. Archived from the original on 2015-09-20.
- Gerdes H, Ferguson MK (2002). "Palliation of Esophageal Cancer". In Posner MC, Vokes EE, Weichselbaum RR (eds.). Cancer of the Upper Gastrointestinal Tract. PMPH-USA. p. 184. ISBN 978-1-55009-101-4. Archived from the original on 2015-10-30.
- ^ Lao-Sirieix P, Caldas C, Fitzgerald RC (June 2010). "Genetic predisposition to gastro-oesophageal cancer". Current Opinion in Genetics & Development. 20 (3): 210–217. doi:10.1016/j.gde.2010.03.002. PMID 20347291.
- Tobias JS, Hochhauser D (2013). Cancer and its management (6th ed.). John Wiley & Sons. p. 254. ISBN 978-1-118-71325-9.
- Prabhu A, Obi KO, Rubenstein JH (June 2014). "The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: a meta-analysis". The American Journal of Gastroenterology. 109 (6): 822–827. doi:10.1038/ajg.2014.71. PMID 24751582. S2CID 205103765.
- Loomis D, Guyton KZ, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. (International Agency for Research on Cancer Monograph Working) (July 2016). "Carcinogenicity of drinking coffee, mate, and very hot beverages" (PDF). The Lancet. Oncology. 17 (7): 877–878. doi:10.1016/s1470-2045(16)30239-x. PMID 27318851. Archived from the original (PDF) on 2016-10-05. Retrieved 2016-10-03.
- "Q&A on Monographs Volume 116: Coffee, maté, and very hot beverages" (PDF). www.iarc.fr. IARC / WHO. Archived (PDF) from the original on 5 July 2016. Retrieved 3 October 2016.
- Jobe BA, Thomas CR, Hunter JG, eds. (2009). Esophageal cancer principles and practice. New York: Demos Medical. p. 93. ISBN 978-1-935281-17-7. Archived from the original on 2017-09-10.
- ^ Rutegård M, Lagergren P, Nordenstedt H, Lagergren J (July 2011). "Oesophageal adenocarcinoma: the new epidemic in men?". Maturitas. 69 (3): 244–248. doi:10.1016/j.maturitas.2011.04.003. PMID 21602001.
- ^ de Jonge PJ, van Blankenstein M, Grady WM, Kuipers EJ (January 2014). "Barrett's oesophagus: epidemiology, cancer risk and implications for management". Gut. 63 (1): 191–202. doi:10.1136/gutjnl-2013-305490. hdl:1765/67455. PMC 6597262. PMID 24092861.
- Janmaat VT, Nesteruk K, Spaander MC, Verhaar AP, Yu B, Silva RA, et al. (June 2021). "HOXA13 in etiology and oncogenic potential of Barrett's esophagus". Nature Communications. 12 (1): 3354. Bibcode:2021NatCo..12.3354J. doi:10.1038/s41467-021-23641-8. PMC 8184780. PMID 34099670.
- Režen T, Rozman D, Kovács T, Kovács P, Sipos A, Bai P, Mikó E (April 2022). "The role of bile acids in carcinogenesis". Cellular and Molecular Life Sciences. 79 (5): 243. doi:10.1007/s00018-022-04278-2. PMC 9013344. PMID 35429253.
- Turati F, Tramacere I, La Vecchia C, Negri E (March 2013). "A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma". Annals of Oncology. 24 (3): 609–617. doi:10.1093/annonc/mds244. PMID 22898040.
- ^ Lagergren J (June 2011). "Influence of obesity on the risk of esophageal disorders". Nature Reviews. Gastroenterology & Hepatology. 8 (6): 340–347. doi:10.1038/nrgastro.2011.73. PMID 21643038. S2CID 31598439.
- ^ Lagergren J, Lagergren P (2013). "Recent developments in esophageal adenocarcinoma". CA. 63 (4): 232–248. doi:10.3322/caac.21185. PMID 23818335.
- ^ Falk GW (July 2009). "Risk factors for esophageal cancer development" (PDF). Surgical Oncology Clinics of North America. 18 (3): 469–485. doi:10.1016/j.soc.2009.03.005. PMID 19500737. Archived (PDF) from the original on 2014-08-12.
- Harris RE (2013). "Epidemiology of Esophageal Cancer". Epidemiology of Chronic Disease: Global Perspectives. Burlington, MA: Jones & Bartlett Publishers. pp. 157–161. ISBN 978-0-7637-8047-0. Archived from the original on 2015-09-25.
- Priante AV, Castilho EC, Kowalski LP (April 2011). "Second primary tumors in patients with head and neck cancer". Current Oncology Reports. 13 (2): 132–137. doi:10.1007/s11912-010-0147-7. PMID 21234721. S2CID 207335139.
- Scherübl H, Steinberg J, Schwertner C, Mir-Salim P, Stölzel U, de Villiers EM (June 2008). "" [Coincidental squamous cell cancers of the esophagus, head, and neck: risk and screening]. Hno (in German). 56 (6): 603–608. doi:10.1007/s00106-007-1616-7. PMID 17928979. S2CID 9504791.
- "Tylosis with esophageal cancer". rarediseases.info.nih.gov. Genetic and Rare Diseases Information Center (GARD) – NIH. 18 January 2013. Archived from the original on 19 August 2014. Retrieved 16 August 2014.
- Nyrén O, Adami HO (2008). "Esophageal Cancer". In Adami HO, Hunter DJ, Trichopoulos D (eds.). Textbook of Cancer Epidemiology. Vol. 1. Oxford University Press. p. 224. ISBN 978-0-19-531117-4. Archived from the original on 2015-10-25.
- Liyanage SS, Rahman B, Ridda I, Newall AT, Tabrizi SN, Garland SM, et al. (2013). "The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis". PLOS ONE. 8 (7): e69238. Bibcode:2013PLoSO...869238L. doi:10.1371/journal.pone.0069238. PMC 3722293. PMID 23894436.
- Sitas F, Egger S, Urban MI, Taylor PR, Abnet CC, Boffetta P, et al. (January 2012). "InterSCOPE study: Associations between esophageal squamous cell carcinoma and human papillomavirus serological markers". Journal of the National Cancer Institute. 104 (2): 147–158. doi:10.1093/jnci/djr499. PMC 3260131. PMID 22228147.
- Syrjänen K (January 2013). "Geographic origin is a significant determinant of human papillomavirus prevalence in oesophageal squamous cell carcinoma: systematic review and meta-analysis". Scandinavian Journal of Infectious Diseases. 45 (1): 1–18. doi:10.3109/00365548.2012.702281. PMID 22830571. S2CID 22862509.
- Hardefeldt HA, Cox MR, Eslick GD (June 2014). "Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis". Epidemiology and Infection. 142 (6): 1119–1137. doi:10.1017/S0950268814000016. PMC 9151180. PMID 24721187. S2CID 21457534.
- Han Y, Chen W, Li P, Ye J (September 2015). "Association Between Coeliac Disease and Risk of Any Malignancy and Gastrointestinal Malignancy: A Meta-Analysis". Medicine. 94 (38): e1612. doi:10.1097/MD.0000000000001612. PMC 4635766. PMID 26402826.
- Sultan R, Haider Z, Chawla TU (January 2016). "Diagnostic accuracy of CT scan in staging resectable esophageal cancer". JPMA. The Journal of the Pakistan Medical Association. 66 (1): 90–92. PMID 26712189.
- Kim TJ, Kim HY, Lee KW, Kim MS (2009). "Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy". Radiographics. 29 (2): 403–421. doi:10.1148/rg.292085106. PMID 19325056.
- Bruzzi JF, Munden RF, Truong MT, Marom EM, Sabloff BS, Gladish GW, et al. (November 2007). "PET/CT of esophageal cancer: its role in clinical management". Radiographics. 27 (6): 1635–1652. doi:10.1148/rg.276065742. PMID 18025508.
- Linder G, Korsavidou-Hult N, Bjerner T, Ahlström H, Hedberg J (September 2019). "F-FDG-PET/MRI in preoperative staging of oesophageal and gastroesophageal junctional cancer". Clinical Radiology. 74 (9): 718–725. doi:10.1016/j.crad.2019.05.016. PMID 31221468. S2CID 80059143.
- Shields TW, LoCicero JW, Reed CE, Feins RH (2009). General Thoracic Surgery. Lippincott Williams & Wilkins. pp. 2047–. ISBN 978-0-7817-7982-1. Archived from the original on 2015-10-25.
- Halperin EC, Perez CA, Brady LW (2008). Perez and Brady's Principles and Practice of Radiation Oncology. Lippincott Williams & Wilkins. pp. 1137–. ISBN 978-0-7817-6369-1. Archived from the original on 2015-10-19.
- Cancer arising at the junction between the esophagus and stomach is often classified as stomach cancer, as in ICD-10. See: "C16 - Malignant neoplasm of the stomach". ICD-10 Version: 2015. World Health Organization. Archived from the original on 2 November 2015. Retrieved 14 November 2014.
- Rice TW, Blackstone EH, Rusch VW (March 2010). "A cancer staging primer: esophagus and esophagogastric junction". The Journal of Thoracic and Cardiovascular Surgery. 139 (3): 527–529. doi:10.1016/j.jtcvs.2009.11.002. PMID 20176201.
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K (August 2016). "Body Fatness and Cancer--Viewpoint of the IARC Working Group". The New England Journal of Medicine. 375 (8): 794–798. doi:10.1056/NEJMsr1606602. PMC 6754861. PMID 27557308.
- Qin X, Jia G, Zhou X, Yang Z (December 2022). "Diet and Esophageal Cancer Risk: An Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies". Advances in Nutrition. 13 (6): 2207–2216. doi:10.1093/advances/nmac087. PMC 9776643. PMID 36041184.
- Chainani-Wu N (2002). "Diet and oral, pharyngeal, and esophageal cancer". Nutrition and Cancer. 44 (2): 104–126. doi:10.1207/S15327914NC4402_01. PMID 12734057. S2CID 1546319. Archived from the original on 2008-04-30.
- Coleman HG, Murray LJ, Hicks B, Bhat SK, Kubo A, Corley DA, et al. (July 2013). "Dietary fiber and the risk of precancerous lesions and cancer of the esophagus: a systematic review and meta-analysis". Nutrition Reviews. 71 (7): 474–482. doi:10.1111/nure.12032. PMID 23815145.
- ^ Zhang Y (September 2013). "Epidemiology of esophageal cancer". World Journal of Gastroenterology. 19 (34): 5598–5606. doi:10.3748/wjg.v19.i34.5598. PMC 3769895. PMID 24039351.
- Dunbar KB, Spechler SJ (July 2014). "Controversies in Barrett esophagus". Mayo Clinic Proceedings. 89 (7): 973–984. doi:10.1016/j.mayocp.2014.01.022. PMID 24867396.
- ^ Tobias JS, Hochhauser D (2010). Cancer and its Management (6th ed.). John Wiley & Sons. p. 257. ISBN 978-1-118-71325-9.
- Berry 2014, p. S292
- Fernández-Esparrach G, Calderón A, de la Peña J, Díaz Tasende JB, Esteban JM, Gimeno-García AZ, et al. (April 2014). "Endoscopic submucosal dissection". Endoscopy. 46 (4): 361–370. doi:10.1055/s-0034-1364921. PMID 24671864.
- Sun F, Yuan P, Chen T, Hu J (May 2014). "Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis". Journal of Cardiothoracic Surgery. 9: 78. doi:10.1186/1749-8090-9-78. PMC 4052291. PMID 24885614.
- ^ Mendenhall WM, Werning JW, Pfister DG (2011). "Ch Chapter 72: Treatment of head and neck cancer". In DeVita Jr VT, Lawrence TS, Rosenberg SA (eds.). DeVita, Hellman, and Rosenberg's Cancer: Cancer: Principles & Practice of Oncology (9th ed.). Philadelphia, Pa: Lippincott Williams & Wilkins. pp. 729–780. ISBN 978-1-4511-0545-2. Online edition, with updates to 2014
- Berry MF (May 2014). "Esophageal cancer: staging system and guidelines for staging and treatment". Journal of Thoracic Disease. 6 (Suppl 3): S289–S297. doi:10.3978/j.issn.2072-1439.2014.03.11. PMC 4037413. PMID 24876933.
- Parameswaran R, McNair A, Avery KN, Berrisford RG, Wajed SA, Sprangers MA, Blazeby JM (September 2008). "The role of health-related quality of life outcomes in clinical decision making in surgery for esophageal cancer: a systematic review". Annals of Surgical Oncology. 15 (9): 2372–2379. doi:10.1245/s10434-008-0042-8. PMID 18626719. S2CID 19933001.
- Berry 2014, p. S293
- Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P, et al. (April 2002). "Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer". Journal of Clinical Oncology. 20 (8): 1996–2004. doi:10.1200/JCO.2002.08.105. PMID 11956258.
- Wang KK, Prasad G, Tian J (September 2010). "Endoscopic mucosal resection and endoscopic submucosal dissection in esophageal and gastric cancers". Current Opinion in Gastroenterology. 26 (5): 453–458. doi:10.1097/MOG.0b013e32833e4712. PMC 3215503. PMID 20703112.
- Polednak AP (May 2003). "Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas". International Journal of Cancer. 105 (1): 98–100. doi:10.1002/ijc.11029. PMID 12672037. S2CID 6539230.
- "Oesophageal cancer survival statistics". Cancer Research UK. 2015-05-15. Archived from the original on 2014-10-08.
- "Esophageal Cancer Treatment | How We Treat Esophageal Cancer | KAIZEN Hospital". www.kaizenhospital.com. Archived from the original on 2021-01-21. Retrieved 2021-01-17.
- ^ Conteduca V, Sansonno D, Ingravallo G, Marangi S, Russi S, Lauletta G, Dammacco F (August 2012). "Barrett's esophagus and esophageal cancer: an overview". International Journal of Oncology. 41 (2): 414–424. doi:10.3892/ijo.2012.1481. PMID 22615011. Archived from the original on 2013-05-17.
- ^ Napier KJ, Scheerer M, Misra S (May 2014). "Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities". World Journal of Gastrointestinal Oncology. 6 (5): 112–120. doi:10.4251/wjgo.v6.i5.112. PMC 4021327. PMID 24834141.
- ^ Arnold M, Soerjomataram I, Ferlay J, Forman D (March 2015). "Global incidence of oesophageal cancer by histological subtype in 2012". Gut. 64 (3): 381–387. doi:10.1136/gutjnl-2014-308124. PMID 25320104.
- Kachala R (September 2010). "Systematic review: epidemiology of oesophageal cancer in Sub-Saharan Africa". Malawi Medical Journal. 22 (3): 65–70. doi:10.4314/mmj.v22i3.62190. PMC 3345777. PMID 21977849.
- ^ "Cancer Facts and Figures 2014" (PDF). American Cancer Society. Archived (PDF) from the original on 29 April 2014. Retrieved 28 April 2014.
- Vega KJ, Jamal MM, Wiggins CL (June 2010). "Changing pattern of esophageal cancer incidence in New Mexico: a 30-year evaluation". Digestive Diseases and Sciences. 55 (6): 1622–1626. doi:10.1007/s10620-009-0918-x. PMC 2882567. PMID 19688596.
- "Incidence and Mortality Rate Trends" (PDF). A Snapshot of Esophageal Cancer. National Cancer Institute. September 2006. Archived from the original (PDF) on 2007-03-16. Retrieved 2007-03-21.
- "Oesophageal cancer statistics". Cancer Research UK. 2015-05-14. Archived from the original on 6 October 2014. Retrieved 3 October 2014.
- "Christopher Hitchens' widow on his death: "God never came up"". CBS News. 7 September 2012. Archived from the original on 30 October 2015. Retrieved 11 November 2015.
- "Morrissey Talks Trump, Cancer Diagnosis, TSA Groping With Larry King". Rolling Stone. 2015-08-19. Archived from the original on 9 December 2015. Retrieved 11 November 2015.
- Sun L, Yu S (November 2011). "Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma". Diseases of the Esophagus. 24 (8): 544–549. doi:10.1111/j.1442-2050.2011.01198.x. PMID 21539676.
- Frankell AM, Jammula S, Li X, Contino G, Killcoyne S, Abbas S, et al. (March 2019). "The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic". Nature Genetics. 51 (3): 506–516. doi:10.1038/s41588-018-0331-5. PMC 6420087. PMID 30718927.
- ^ M Naeini M, Newell F, Aoude LG, Bonazzi VF, Patel K, Lampe G, et al. (May 2023). "Multi-omic features of oesophageal adenocarcinoma in patients treated with preoperative neoadjuvant therapy". Nature Communications. 14 (1): 3155. Bibcode:2023NatCo..14.3155M. doi:10.1038/s41467-023-38891-x. PMC 10232490. PMID 37258531.
External links
| Classification | D |
|---|---|
| External resources |
- NCI esophageal cancer
- Cancer.Net: Esophageal Cancer
- Esophageal Cancer Archived 2009-07-12 at the Wayback Machine From Cancer Management: A Multidisciplinary Approach Archived 2009-05-15 at the Wayback Machine
- Oesophageal Cancer at Cancer Research UK
- National Comprehensive Cancer Network Archived 2019-03-31 at the Wayback Machine