
| |
| Names | |
|---|---|
| IUPAC name Estra-1,3,5(10)-triene | |
| Systematic IUPAC name (3aS,3bS,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopentaphenanthrene | |
| Other names Estratriene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C18H24 |
| Molar mass | 240.39 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Estrin (American English), or oestrin (British English), also known as estra-1,3,5(10)-triene, is an estrane steroid. It is dehydrogenated estrane with double bonds specifically at the C1, C3, and C5(10) positions. Estrin is a parent structure of the estrogen steroid hormones estradiol, estrone, and estriol, which have also been known as dihydroxyestrin, ketohydroxyestrin, and trihydroxyestrin, respectively.
Unlike its estrogen derivatives, estrin itself possesses minimal estrogenic activity, as hydroxyl and/or keto substitutions at the C3 and C17 positions are critical for high binding affinity to the estrogen receptors. Estrin has been found to be on the order of 1,000-fold less potent than estradiol in inducing estrogenic responses in vitro. In addition to estrin, estratrien-17β-ol, which lacks the 3-hydroxyl group of estradiol, and 3-hydroxyestratriene, which lacks the 17β-hydroxyl group of estradiol, both have measurable affinity for the estrogen receptor and are able to activate the receptor and induce progesterone receptor expression.
The term estrin is also a synonym for estrogen. It was coined by Sir Alan S. Parkes and C. W. Bellerby in 1926 to describe the hormone secreted from the ovaries that induces estrus in animals (i.e., estrogen).
Structures of major endogenous estrogens
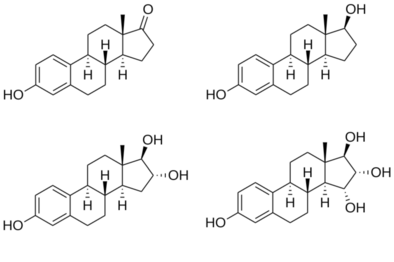 Estrone (E1)
Estradiol (E2)
Estriol (E3)
Estetrol (E4)
Estrone (E1)
Estradiol (E2)
Estriol (E3)
Estetrol (E4)
|
See also
References
- Biskind, Morton S. (1935). "Commercial glandular products". Journal of the American Medical Association. 105 (9): 667. doi:10.1001/jama.1935.92760350007009a. ISSN 0002-9955.
- Fluhmann CF (1938). "Estrogenic Hormones: Their Clinical Usage". Cal West Med. 49 (5): 362–6. PMC 1659459. PMID 18744783.
- ^ Anstead GM, Carlson KE, Katzenellenbogen JA (1997). "The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site". Steroids. 62 (3): 268–303. doi:10.1016/s0039-128x(96)00242-5. PMID 9071738. S2CID 10080044.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1395–. ISBN 978-1-60913-345-0.
- Jordan VC, Koch R (April 1989). "Regulation of prolactin synthesis in vitro by estrogenic and antiestrogenic derivatives of estradiol and estrone". Endocrinology. 124 (4): 1717–26. doi:10.1210/endo-124-4-1717. PMID 2924721.
- ^ Brooks SC, Wappler NL, Corombos JD, Doherty LM, Horwitz JP (1987). "Estrogen structure-receptor function relationships". In Moudgil VK (ed.). Recent Advances in Steroid Hormone Action. Walter de Gruyter. pp. 443–466.
- Schwartz JA, Skafar DF (September 1993). "Ligand-mediated modulation of estrogen receptor conformation by estradiol analogs". Biochemistry. 32 (38): 10109–15. doi:10.1021/bi00089a029. PMID 8399136.
- ^ Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 750–. ISBN 978-1-4511-4847-3.
| Steroid classification | |
|---|---|
| C17 | |
| C18 |
|
| C19 | |
| C20 | |
| C21 |
|
| C23 | |
| C24 | |
| C27 | |
| Functional group | |
| Elements removed | |
| Elements replaced | |
This article about a steroid is a stub. You can help Misplaced Pages by expanding it. |