
| |
| Names | |
|---|---|
| IUPAC name Abieta-8,11,13-trien-12-ol | |
| Systematic IUPAC name (4bS,8aS)-4b,8,8-Trimethyl-2-(propan-2-yl)-4b,5,6,7,8,8a,9,10-octahydrophenanthren-3-ol | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H30O |
| Molar mass | 286.459 g·mol |
| Density | 1.0±0.1 g/cm |
| Melting point | 56-57 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ferruginol is a natural phenol with a terpenoid substructure. Specifically, it is a diterpene of the abietane chemical class, meaning it is characterized by three fused six-membered rings and alkyl functional groups. Ferruginol was first identified in 1939 by Brandt and Neubauer as the main component in the resin of the Miro tree (Podocarpus ferrugneus) and has since been isolated from other conifer species in the families Cupressaceae and Podocarpaceae. As a biomarker, the presence of ferruginol in fossils, mainly resin, is used to describe the density of these conifers in that particular biosphere throughout time.
Background
Ferruginol is a phenolic abietene, a type of tricyclic diterpenoid derived from terrestrial plants. It has a molecular composition of C20H30O with a molecular weight of 286 g/mole. Along with its presence in the Verbenaceae family, it has been found in a variety of conifer families including Podocarpaceae, the ancient Araucariaceae, and the extinct Cheirolepidiaceae. It is particularly useful as a biomarker because of its concentration in the Cupressaceae family. In these conifers, it acts as a plant metabolite, along with some protective and antibacterial roles.
Preservation
As a polar terpenoid, ferruginol was thought to have poor preservation potential. However, the discovery of resin fossils provided unaltered natural diterpenoids that can be used to understand botanical diversity during a given geological age. Analysis of macrofossils or clay sediments is also used to identify the presence of ferruginol, though these samples may not be fully preserved. Comparing the composition of fossilized coal or clay from the same region as resin fossils can indicate the original biological precursors of these samples. Additionally, the diagenetic alterations of fossils can be used to understand the environmental changes in the time after they were formed. General abietane diterpenoid abietic acids have been connected to the diagenetic products simonellite and retene. Microbial and abiotic degradation make it so most conifer biomarkers cannot be linked to specific species, so it is especially useful to find resinous samples that are able to provide more detailed identification.
Due to the improved preservation of ferruginol and other diterpenoids in fossil resin, they have been found to be underrepresented in sediment samples when compared to angiosperms, whose leaf waxes are more free to disperse throughout the sample. Even in regions known to have a high abundance of conifers, sediment samples have been found to contain little to no unaltered diterpenoids. The relative of abundance of conifers cannot therefore be directly determined from biomarker concentrations in sediment samples, as this will be biased by preservation.
The presence of ferruginol has also been used in more modern samples as biological tracers. For example, analyzing the honeybee product propolis helps establish the main botanical source collected by the bees.
Measurement
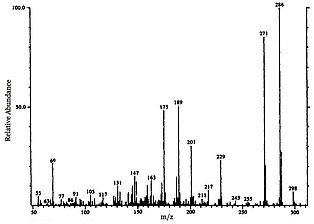
The sample preparation to measure ferruginol abundance varies depending on form the sample initially takes, though generally follows the same structure. After physically crushing the sample, N,O-bistrifluoroacetamide (BSTFA) is used to transform molecules into trimethyl-silyl (TMS) derivatives. It is then chemically extracted into neutral, aromatic, and polar fractions using specified eluents, often hexane, dichloromethane, and methanol, respectively. The aromatic fractions are then analyzed using gas chromatography–mass spectrometry (GC-MS), and library data along with fragmentation patterns are used to identify the molecular makeup of each notable peak and their relative concentration in the sample. Ferruginol can be identified with a molecular weight of 286 m/z.
Along with GC-MS, ferruginol has also been analyzed using cross polarization/magic angle spinning nuclear magnetic resonance (3C-CPMAS-NMR) to provide more detailed analysis. Additionally, time-of-flight secondary ion mass spectrometry (TOF-SIMS) has been used in combination with GC-MS with samples collected from still living organisms for surface imaging and depth profiling.
Bioactivity
Research published in 2005 found that this and other compounds of the class from Sequoia have in vitro anti-tumor and anti-inflammatory properties in cell lines. In vitro studies have shown human colon, breast, and lung tumor reduction and reduction in oncogene transformed cells as well. Ferruginol has also been found to have antibacterial activity and gastroprotective effects.
When studied against human prostate cancer cells, ferruginol induced cell death by suppressing survival signaling pathways. Specific activity of tumor growth inhibition (GI) is 2-5 micrograms/milliliter. Beyond anti-cancer activity, studies with mice showed that ferruginol had anti-inflammatory properties against induced ulcerative colitis and acted as a gastroprotective agent against gastro lesions.
Biomarker case study: Brazil
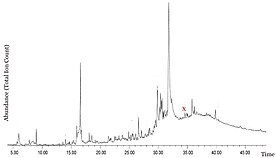
The Araripe Basin in Brazil is well known for the diverse and well-preserved collection of fossils. Despite this, the Ipubi Formation in the central Santana Group is only poorly explored. To better understand the paleoflora, researchers at Universidade Federal Rural de Pernambuco analyzed amber resin from the black shales that make up the collection site. Palynological content had been used to date the Ipubi Formation as Aptian-Albian (125–100.5 mya), and the amber samples were thought to be allochthonous, having swept in from nearby conifer sources. GC-MS analysis resulted in the chromatogram shown to the right, with the ferruginol peak marked in red. Additionally, 3C-CPMAS-NMR was used to further understand the sample. The terpenoids analyzed were separated into three groups: monoterpenes, sesquiterpenoids, and diterpenoids. Diterpenoids of the abietanic class were the most abundant in the amber, though they are widely present in all conifer families and therefore less useful in identifying specific contributing species. The detection of ferruginol helped limit the biological origin to the families Cupressaceae, Podocarpaceae and Cheirolepidiaceae. Further more, the absence callitrisates, kauranes and phyllocladanes excluded Cupressaceae as the source. Therefore, the possible botanical sources of the amber collected in the Ipubi Formation were identified as Podocarpaceae and Cheirolepidiaceae. The results from the amber samples are consistent with environmental conditions determined from a separate analysis of the bituminous shale.
References
- Brandt, C. W.; Neubauer, L. G. (1939-01-01). "221. Miro resin. Part I. Ferruginol". Journal of the Chemical Society (Resumed): 1031–1037. doi:10.1039/JR9390001031. ISSN 0368-1769.
- ^ Otto, Angelika; Simoneit, Bernd R.T.; Rember, William C. (2005-06-01). "Conifer and angiosperm biomarkers in clay sediments and fossil plants from the Miocene Clarkia Formation, Idaho, USA". Organic Geochemistry. 36 (6): 907–922. Bibcode:2005OrGeo..36..907O. doi:10.1016/j.orggeochem.2004.12.004. ISSN 0146-6380.
- ^ Stefanova, Maya; Simoneit, Bernd R.T. (2008-08-05). "Polar aromatic biomarkers of Miocene-aged Chukurovo resinite and correlation with a progenitor macrofossil". International Journal of Coal Geology. 75 (3): 166–174. Bibcode:2008IJCG...75..166S. doi:10.1016/j.coal.2008.05.003. ISSN 0166-5162.
- Otto, A.; Walther, H.; Püttmann, W. (1997-01-01). "Sesqui- and diterpenoid biomarkers preserved in Taxodium-rich Oligocene oxbow lake clays, Weisselster basin, Germany". Organic Geochemistry. 26 (1–2): 105–115. Bibcode:1997OrGeo..26..105O. doi:10.1016/S0146-6380(96)00133-7. ISSN 0146-6380.
- Popova, Milena; Trusheva, Boryana; Cutajar, Simone; Antonova, Daniela; Mifsud, David; Farrugia, Claude; Bankova, Vassya (May 2012). "Identification of the Plant Origin of the Botanical Biomarkers of Mediterranean type Propolis". Natural Product Communications. 7 (5): 569–570. doi:10.1177/1934578X1200700505. ISSN 1934-578X. PMID 22799077.
- ^ Pereira, Ricardo; Lima, Flaviana Jorge de; Simbras, Felipe M.; Bittar, Sheila Maria Bretas; Kellner, Alexander Wilhelm Armin; Saraiva, Antônio Álamo F.; Bantim, Renan A.M.; Sayão, Juliana M.; Oliveira, Gustavo R. (2020-03-01). "Biomarker signatures of Cretaceous Gondwana amber from Ipubi Formation (Araripe Basin, Brazil) and their palaeobotanical significance". Journal of South American Earth Sciences. 98: 102413. Bibcode:2020JSAES..9802413P. doi:10.1016/j.jsames.2019.102413. ISSN 0895-9811. S2CID 210270723.
- Imai, Takanori; Tanabe, Kinuko; Kato, Toshiyuki; Fukushima, Kazuhiko (2005-06-01). "Localization of ferruginol, a diterpene phenol, in Cryptomeria japonica heartwood by time-of-flight secondary ion mass spectrometry". Planta. 221 (4): 549–556. doi:10.1007/s00425-004-1476-2. ISSN 1432-2048. PMID 15856284. S2CID 6298474.
- E.C.J. Smith; G.W. Kaatz; E.M. Williamson; S. Gibbons. "P-168: The Resistance Modifying Activity of Ferruginol" (PDF). Archived from the original (PDF) on October 9, 2007.
{{cite journal}}: Cite journal requires|journal=(help) - C. Flores; J. Alarcón; J. Becerra; M. Bittner; M. Hoeneisen; M. Silva (2001). "EXTRACTABLE COMPOUNDS OF NATIVE TREES: CHEMICAL AND BIOLOGICAL STUDY I: Bark of Prumnopytis andina (Podocarpaceae) and Austrocedrus chilensis (Cupressaceae)". Bol. Soc. Chil. Quím. 46 (1). doi:10.4067/S0366-16442001000100010.
- Areche, Carlos; Theoduloz, Cristina; Yáñez, Tania; Souza-Brito, Alba R. M.; Barbastefano, VíCtor; De Paula, DéBora; Ferreira, Anderson L.; Schmeda-Hirschmann, Guillermo; Rodríguez, Jaime A. (2008). "Gastroprotective activity of ferruginol in mice and rats: effects on gastric secretion, endogenous prostaglandins and non-protein sulfhydryls". Journal of Pharmacy and Pharmacology. 60 (2): 245–51. doi:10.1211/jpp.60.2.0014. hdl:10533/139107. PMID 18237473. S2CID 9928974.
- Bispo De Jesus, Marcelo; Zambuzzi, Willian Fernando; Ruela De Sousa, Roberta Regina; Areche, Carlos; Santos De Souza, Ana Carolina; Aoyama, Hiroshi; Schmeda-Hirschmann, Guillermo; Rodríguez, Jaime A.; Monteiro De Souza Brito, Alba Regina; Peppelenbosch, Maikel P.; Den Hertog, Jeroen; De Paula, Eneida; Ferreira, Carmen Veríssima (2008-06-01). "Ferruginol suppresses survival signaling pathways in androgen-independent human prostate cancer cells". Biochimie. 90 (6): 843–854. doi:10.1016/j.biochi.2008.01.011. ISSN 0300-9084. PMID 18294971.
- Son, Kh; Oh, Hm; Choi, Sk; Han, Dc; Kwon, Bm (Apr 2005). "Anti-tumor abietane diterpenes from the cones of Sequoia sempervirens". Bioorganic & Medicinal Chemistry Letters. 15 (8): 2019–21. doi:10.1016/j.bmcl.2005.02.057. PMID 15808460.
- Zhu, Xiao-Yan; Zhang, Chun-Ling; Lin, Yukiat; Dang, Min-Yan (2020). "Ferruginol alleviates inflammation in dextran sulfate sodium-induced colitis in mice through inhibiting COX-2, MMP-9 and NF-κB signaling". Asian Pacific Journal of Tropical Biomedicine. 10 (7): 308. doi:10.4103/2221-1691.284945. ISSN 2221-1691.
- Areche, Carlos; Theoduloz, Cristina; Yáñez, Tania; Souza-Brito, Alba R M; Barbastefano, Víctor; de Paula, Débora; Ferreira, Anderson L; Schmeda-Hirschmann, Guillermo; Rodríguez, Jaime A (2008-02-01). "Gastroprotective activity of ferruginol in mice and rats: effects on gastric secretion, endogenous prostaglandins and non-protein sulfhydryls†". Journal of Pharmacy and Pharmacology. 60 (2): 245–251. doi:10.1211/jpp.60.2.0014. ISSN 0022-3573. PMID 18237473. S2CID 9928974.