 Parkinsonian gait (indoor)
Parkinsonian gait (indoor) Parkinsonian gait (outdoor)Features of PD gait are more obvious in the side view.
Parkinsonian gait (outdoor)Features of PD gait are more obvious in the side view.
Parkinsonian gait (or festinating gait, from Latin festinare ) is the type of gait exhibited by patients with Parkinson's disease (PD). It is often described by people with Parkinson's as feeling like being stuck in place, when initiating a step or turning, and can increase the risk of falling. This disorder is caused by a deficiency of dopamine in the basal ganglia circuit leading to motor deficits. Gait is one of the most affected motor characteristics of this disorder although symptoms of Parkinson's disease are varied.
Parkinsonian gait is characterized by small shuffling steps and a general slowness of movement (hypokinesia), or even the total loss of movement (akinesia) in extreme cases. Patients with PD demonstrate reduced stride length, walking speed during free ambulation and cadence rate, while double support duration is increased. The patient has difficulty starting, but also has difficulty stopping after starting. This is due to muscle hypertonicity.
Abnormal gait characteristics

Patients with Parkinson's disease exhibit gait characteristics that are markedly different from normal gait. While the list of abnormal gait characteristics given below is the most discussed, it is certainly not exhaustive.
Heel to toe characteristics
Whereas in normal gait, the heel strikes the ground before the toes (also called heel-to-toe walking), in Parkinsonian gait, motion is characterised by flat foot strike (where the entire foot is placed on the ground at the same time) or less often and in the more advanced stages of the disease by toe-to-heel walking (where the toes touch the ground before the heel). In addition, PD patients have reduced foot lifting during the swing phase of gait, which produces smaller clearance between the toes and the ground.
Patients with Parkinson's disease have reduced impact at heel strike and this mechanism has been found to be related to the disease severity with impact decreasing as the disease progresses. Also, Parkinson patients show a trend towards higher relative loads in the forefoot regions combined with a load shift towards medial foot areas. This load shift is believed to help in compensating for postural imbalance. The intra-individual variability in foot strike pattern is found to be surprisingly lower in PD patients compared with those with a typical gait.
Vertical ground reaction force
In normal gait, the vertical ground reaction force (GRF) plot has two peaks – one when the foot strikes the ground and the second peak is caused by push-off force from the ground. The shape of the vertical GRF signal is abnormal in PD. In the earlier stages of the disease, reduced forces (or peak heights) are found for heel contact and the push-off phase resembling that of elderly subjects. In the more advanced stages of the disorder where gait is characterized by small shuffling steps, PD patients show only one narrow peak in the vertical GRF signal.
Falls and freezing of gait
Falls and freezing of gait are two episodic phenomena that are common in Parkinsonian gait. Falls and freezing of gait in PD are generally thought to be closely intertwined for several reasons, most importantly: both symptoms are common in the advanced stages of the disease and are less common in the earlier stages, with freezing of gait leading to falls in many instances. Both symptoms often respond poorly and sometimes paradoxically to treatment with dopaminergic medication, perhaps pointing to a common underlying pathophysiology. It is possible to demonstrate poor and paradoxical dopaminergic medication response through a challenge paradigm in which gait is assessed after withdrawal from medication and in the full ON medication state.
Freezing of Gait: Freezing of Gait (FOG) is typically a transient episode – lasting less than a minute, in which gait is halted and the patient complains that his/her feet are glued to the ground. When the patient overcomes the block, walking can be performed relatively smoothly. The pathophysiology of the phenomenon is poorly understood but likely extends across a disseminated functional-anatomic network. Current treatments for FOG offer only limited benefits but a range of novel approaches are being actively explored, and thought is being given to how future research strategies are best coordinated.
The most common form of FOG is 'start hesitation' (which happens when the patient wants to start walking) followed in frequency by 'turning hesitation' FOG can also be experienced in narrow or tight quarters such as a doorway, whilst adjusting one's steps when reaching a destination, and in stressful situations such as when the telephone or the doorbell rings or when the elevator door opens. As the disease progresses, FOG can appear spontaneously even in an open runway space. It is proven that psychological interventions can help reduce negative effect of psychosocial factors, like anxiety or depression, that can worsen freezing of gait or tremor in Parkinson's patients. Based on that, every patient could benefit from psychological intervention, not only to reduce anxiety, depression, pain, and insomnia, but also to reduce effect of psychosocial factors in worsening of motor symptoms.
Falls: Falls, like FOG are rare in the earlier stages of the disorder and becomes more frequent as the disease progresses. Falls result mainly due to sudden changes in posture, in particular turning movements of the trunk, or attempts to perform more than one activity simultaneously with walking or balancing. Falls are also common during transfers, such as rising from a chair or bed. PD patients fall mostly forward (45% of all falls) and about 20% fall laterally. Falls that occur frequently very early in the disease course may signify that another diagnosis (such as progressive supranuclear palsy) should be considered.
Postural sway
Postural instability in upright stance is common in end-stage PD and compromises the ability to maintain balance during everyday tasks such as walking, turning and standing up from sitting. An inability to adequately balance the body's center of mass over the base of support combined with inflexibility in body movements (due to increased rigidity) causes patients with advanced PD to fall. Whereas postural sway in normal stance usually increases in patients with balance disorders arising from stroke, head injury and cerebellar ataxia it is often reduced in patients with PD. This is because in PD, the problem appears to be a lack of flexibility in shifting postural responses. This inflexibility increases the tendency to fall in these patients.
Electromyographic studies
Electromyographic (EMG) studies of the leg muscles in PD patients have shown an extreme reduction in the activation of the tibialis anterior muscle in the early stance and in the early and late swing phases, and a reduction in triceps surae muscle bursting at push-off. The quadriceps and hamstring muscles on the other hand, show prolonged activation in the stance phase of gait. This implies that PD patients have higher passive stiffness of ankle joints, show larger background EMG activity and more co-contraction of leg muscles in stance. Stiffer joints lead to abnormal postural sway in the PD patients.
Gait improvement strategies
Drugs
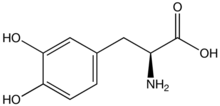
The most widely used form of treatment is L-dopa in various forms. L-dopa is able to pass the blood–brain barrier as a prodrug and is decarboxylated in the brain to the neurotransmitter dopamine by the enzyme aromatic-L-amino-acid decarboxylase. In this way, L-DOPA can replace some of the deficit in dopamine seen in Parkinsonism. Due to feedback inhibition, L-dopa results in a reduction in the endogenous formation of L-dopa, and so eventually becomes counterproductive.
Effect on gait parameters: The stride length and the kinematic parameters (swing velocity, peak velocity) related to the energy are Dopa-sensitive. Temporal parameters (stride and swing duration, stride duration variability), related to rhythm, are Dopa-resistant.
Effect on falls and freezing of gait: Levodopa treatment decreases the frequency and the akinetic type of FOG, with a tendency for shorter FOG episodes. Results indicate that this is primarily because L-dopa increases the threshold for FOG to occur but the fundamental pathophysiology for FOG did not change. It has also been shown that other dopamine agonists like ropinirole, pramipexole and pergolide that have a strong affinity to D2 receptors (as opposed to L-dopa which has a strong D1 receptor affinity) increase the frequency of FOGs.
Effects on postural sway: Parkinson's disease have abnormal postural sway in stance and treatment with levodopa increases postural sway abnormalities. During movement, it has been shown that early autonomic postural disturbances are only partially corrected while the later occurring postural corrections are not affected by dopamine. These results indicate that non-dopaminergic lesions play a role in postural imbalance in PD patients.

Auditory and visual cues
Basal ganglia dysfunction in PD causes it to stop acting as an internal cue for gait in Parkinson's patients. Hence various external sensory cues like auditory and visual cues have been developed to bypass the basal ganglia's cueing functions.
Visual cues: The visual cues are commonly transverse lines or rods on the floor (floor markers). Such cues have been shown to improve stride length and velocity in Parkinsonian gait by substituting kinaesthetic feedback with visual feedback for regulating movement amplitude. In addition gait initiation has been shown to be significantly improved in PD patients compared with auditory cues. Visual cues administered by "laser canes" have been reported to improve gait initiation. Virtual reality glasses have also been developed recently to aid walking in PD patients.
Auditory cues: The auditory cues are commonly rhythmic cues generated by a metronome or equivalent, sometimes embedded in music, set at or slightly above the subject's usual cadence. Rhythmic auditory cues have been associated with increases in velocity and cadence and sometimes stride after gait has been initiated. Auditory cues have been shown to have little or no effect in gait initiation. Moreover, there are prediction algorithms to support more efficient auditory cueing. These algorithms predict freezing episodes so that a cueing can be initiated.
Deep brain stimulation

Deep brain stimulation (DBS) in the pedunculopontine nucleus, a part of the brainstem involved in motor planning, has been shown to improve gait function in patients with Parkinson's disease.
DBS in the subthalamic nucleus (STN) and the globus pallidus have also been shown to have positive effects on gait abnormalities presented by Parkinson's Disease patients. DBS in the STN has been reported to reduce freezing of gait significantly at 1 and 2 year follow up. Contradictory results have been reported on the effects on DBS on postural stability The results seem to be highly location specific. The studies which do report positive effects suggest that the effectiveness of DBS in improving postural stability is due to its ability to affect non-dopaminergic pathways (in addition to dopaminergic pathways) which are believed to cause postural sway in PD patients. Several studies suggest that STN stimulation with low frequencies (60–80 Hz) better alleviates gait deficits than with the commonly used high frequencies (>130 Hz).
Other treatments strategies
Attention strategies: By consciously paying more attention to walking and rehearsing each step before actually making it, PD patients have shown to improve their gait. Sometimes, a companion walking alongside reminds the patient to concentrate on gait or they create a visual cue to step over by putting a foot in front of the person with PD over which the person must step. This causes the patient to focus their attention on the stepping action, thus making this a voluntary action and hence bypassing the faulty basal ganglia pathway (which is responsible for involuntary actions like walking). Avoidance of dual tasks that require motor attention or cognitive attention has also been shown to normalize gait in the PD patients.
Exercise: Physical therapy and exercise have been shown to have positive effects on gait parameters in PD patients.
Physiotherapists may help improve gait by creating training programs to lengthen a patient's stride length, broaden the base of support, improve the heel-toe gait pattern, straighten out a patient's posture, and increase arm swing patterns.
Research has shown gait training combining an overhead harness with walking on a treadmill has shown to improve both walking speed and stride length. The harness assists the patient in maintaining an upright posture by eliminating the need to use a mobility aid, a practice which normally promotes a forward flexed posture. It is believed the activation of the central pattern generator leads to the improvement in gait pattern.
Improving trunk flexibility, along with strengthening of the core muscles and lower extremities has been associated with increased balance and an improvement in gait pattern. Aerobic exercises such as tandem bicycling and water aerobics are also crucial in improving strength and overall balance. Due to PD's progressive nature it is important to sustain an exercise routine to maintain its benefits.
Strategies such as using a vertical walking pole can also help to improve upright postural alignment. The therapist may also use tiles or footprints on the ground to improve foot placement and widen the patient's base of support. Creative visualization of walking with a more normalized gait pattern, and mentally rehearsing the desired movement has also shown to be effective.
The patient should also be challenged by walking on a variety of surfaces such as tile, carpet, grass, or foamed surfaces will also benefit the individual's progress towards normalizing their gait pattern.
Comparison with other gait disorders
Subcortical arteriosclerotic encephalopathy (SAE), also called lower-body parkinsonism, and cerebellar ataxia are two other gait disorders whose symptoms seem to closely resemble that of Parkinson's. However, through regression analysis studies have revealed that in Parkinson's, increasing the velocity of walking changes the stride length linearly (which resembles that of controls). However, in SAE and cerebellar ataxia stride length had a disproportionate contribution to increasing velocity, indicating that SAE and cerebellar ataxia have common underlying mechanisms different from those of Parkinson's.
Socio-economic impact
Mobility issues associated with falls and freezing of gait have a devastating impact in the lives of PD patients. Fear of falling in itself can have an incapacitating effect in PD patients and can result in social seclusion leaving patients largely isolated leading to depression. Immobility can also lead to osteoporosis which in-turn facilitates future fracture development. This then becomes a vicious circle with falls leading to immobility and immobility facilitating future falls. Hip fractures from falls are the most common form of fracture among PD patients. Fractures increase treatment costs associated with health care expenditures in PD. Also, when gait is affected it often heralds the onset of Lewy body dementia.
References
- Connie, Tee; Aderinola, Timilehin B.; Ong, Thian Song; Goh, Michael Kah Ong; Erfianto, Bayu; Purnama, Bedy (12 Dec 2022). "Pose-Based Gait Analysis for Diagnosis of Parkinson's Disease". Algorithms. 15 (12). MDPI AG: 474. doi:10.3390/a15120474. ISSN 1999-4893.
- "Definition: festinating gait from Online Medical Dictionary".
- "Movement Symptoms". Parkinson's Foundation. Retrieved 11 November 2019.
- Morris M, Iansek R, Matyas T, Summers J (January 1998). "Abnormalities in the stride length-cadence relation in parkinsonian gait". Movement Disorders. 13 (1): 61–69. doi:10.1002/mds.870130115. PMID 9452328. S2CID 26059115.
- Aita JF (1982). "Why patients with Parkinson's disease fall". JAMA. 247 (4): 515–516. doi:10.1001/jama.247.4.515. PMID 7054557.
- Koller WC, Glatt S, Vetere-Overfield B, Hassanein R (April 1989). "Falls and Parkinson's disease". Clinical Neuropharmacology. 12 (2): 98–105. doi:10.1097/00002826-198908000-00006. PMID 2720700.
- Morris ME, Iansek R, Matyas TA, Summers JJ (April 1996). "Stride length regulation in Parkinson's disease. Normalization strategies and underlying mechanisms". Brain: A Journal of Neurology. 119 (Pt 2): 551–68. doi:10.1093/brain/119.2.551. PMID 8800948.
- Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL (May 1998). "Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson's disease and Huntington's disease". Movement Disorders. 13 (3): 428–437. doi:10.1002/mds.870130310. PMID 9613733. S2CID 14668413.
- Vieregge P, Stolze H, Klein C, Heberlein I (1997). "Gait quantitation in Parkinson's disease--locomotor disability and correlation to clinical rating scales". Journal of Neural Transmission. 104 (2–3): 237–248. doi:10.1007/bf01273184. PMID 9203085. S2CID 6739090.
- Zijlstra W, Rutgers AW, Van Weerden TW (January 1998). "Voluntary and involuntary adaptation of gait in Parkinson's disease". Gait & Posture. 7 (1): 53–63. doi:10.1016/s0966-6362(97)00037-4. PMID 10200376.
- Saint S, Wiese J, Bent S (2006). Clinical clerkships: the answer book. Hagerstown, MD: Lippincott Williams & Wilkins. p. 218. ISBN 978-0-7817-3754-8.
- Hughes JR, Bowes SG, Leeman AL, O'Neill CJ, Deshmukh AA, Nicholson PW, et al. (February 1990). "Parkinsonian abnormality of foot strike: a phenomenon of ageing and/or one responsive to levodopa therapy?". British Journal of Clinical Pharmacology. 29 (2): 179–186. doi:10.1111/j.1365-2125.1990.tb03617.x. PMC 1380081. PMID 2306409.
- Murray MP, Sepic SB, Gardner GM, Downs WJ (December 1978). "Walking patterns of men with parkinsonism". American Journal of Physical Medicine. 57 (6): 278–294. PMID 742658.
- Kimmeskamp S, Hennig EM (November 2001). "Heel to toe motion characteristics in Parkinson patients during free walking". Clinical Biomechanics (Bristol, Avon). 16 (9): 806–12. doi:10.1016/s0268-0033(01)00069-9. PMID 11714558.
- Koozekanani SH, Balmaseda MT, Fatehi MT, Lowney ED (January 1987). "Ground reaction forces during ambulation in parkinsonism: pilot study". Archives of Physical Medicine and Rehabilitation. 68 (1): 28–30. PMID 3800620.
- Ueno E, Yanagisawa N, Takami M (1993). "Gait disorders in parkinsonism. A study with floor reaction forces and EMG". Advances in Neurology. 60: 414–418. PMID 8420164.
- ^ Bloem BR, Hausdorff JM, Visser JE, Giladi N (August 2004). "Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena". Movement Disorders. 19 (8): 871–884. doi:10.1002/mds.20115. PMID 15300651. S2CID 8867520.
- McKay, J Lucas; Goldstein, FC; Sommerfeld, B; Bernhard, D; Perez Parra, S; Factor, SA (2019-11-22). "Freezing of Gait can persist after an acute levodopa challenge in Parkinson's disease". npj Parkinson's Disease. 5: 25. doi:10.1038/s41531-019-0099-z. PMC 6874572. PMID 31799377.
- Weiss D, Schoellmann A, Fox MD, Bohnen NI, Factor SA, Nieuwboer A, et al. (January 2020). "Freezing of gait: understanding the complexity of an enigmatic phenomenon". Brain. 143 (1): 14–30. doi:10.1093/brain/awz314. PMC 6938035. PMID 31647540.
- Cui CK, Lewis SJ (2 November 2021). "Future Therapeutic Strategies for Freezing of Gait in Parkinson's Disease". Frontiers in Human Neuroscience. 15: 741918. doi:10.3389/fnhum.2021.741918. PMC 8592896. PMID 34795568.
- Lewis S, Factor S, Giladi N, Nieuwboer A, Nutt J, Hallett M (May 2022). "Stepping up to meet the challenge of freezing of gait in Parkinson's disease". Translational Neurodegeneration. 11 (1): 23. doi:10.1186/s40035-022-00298-x. PMC 9057060. PMID 35490252.
- Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, Fahn S (February 1992). "Motor blocks in Parkinson's disease". Neurology. 42 (2): 333–339. doi:10.1212/wnl.42.2.333. PMID 1736161. S2CID 6238658.
- Giladi N, Treves TA, Simon ES, Shabtai H, Orlov Y, Kandinov B, et al. (2001). "Freezing of gait in patients with advanced Parkinson's disease". Journal of Neural Transmission. 108 (1): 53–61. doi:10.1007/s007020170096. PMID 11261746. S2CID 2153896.
- Zečević I (July 2020). "Clinical practice guidelines based on evidence for cognitive-behavioural therapy in Parkinson's disease comorbidities: A literature review". Clinical Psychology & Psychotherapy. 27 (4): 504–514. doi:10.1002/cpp.2448. PMID 32196842. S2CID 214601157.
- Brown, F (2020). "Falls in Progressive Supranuclear Palsy". Movement Disorders Clinical Practice. 7 (1): 16–24. doi:10.1002/mdc3.12879. PMC 6962663. PMID 31970205.
- Morris M, Iansek R, Smithson F, Huxham F (December 2000). "Postural instability in Parkinson's disease: a comparison with and without a concurrent task". Gait & Posture. 12 (3): 205–216. doi:10.1016/s0966-6362(00)00076-x. PMID 11154931.
- Horak FB, Nutt JG, Nashner LM (August 1992). "Postural inflexibility in parkinsonian subjects". Journal of the Neurological Sciences. 111 (1): 46–58. doi:10.1016/0022-510x(92)90111-w. PMID 1402997. S2CID 22710219.
- Cioni M, Richards CL, Malouin F, Bedard PJ, Lemieux R (August 1997). "Characteristics of the electromyographic patterns of lower limb muscles during gait in patients with Parkinson's disease when OFF and ON L-Dopa treatment". Italian Journal of Neurological Sciences. 18 (4): 195–208. doi:10.1007/bf02080464. PMID 9323513. S2CID 23702589.
- Robertson LT, Horak FB, Anderson VC, Burchiel KJ, Hammerstad JP (March 2001). "Assessments of axial motor control during deep brain stimulation in parkinsonian patients". Neurosurgery. 48 (3): 544–551. doi:10.1097/00006123-200103000-00017. PMID 11270544. S2CID 46029068.
- Dietz V, Zijlstra W, Assaiante C, et al. (1993). "Balance control in Parkinson's disease". Gait & Posture. 1 (2): 77–84. doi:10.1016/0966-6362(93)90018-v.
- Blin O, Ferrandez AM, Pailhous J, Serratrice G (May 1991). "Dopa-sensitive and dopa-resistant gait parameters in Parkinson's disease". Journal of the Neurological Sciences. 103 (1): 51–54. doi:10.1016/0022-510x(91)90283-d. PMID 1865232. S2CID 35816130.
- Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N (July 2003). "Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease". European Journal of Neurology. 10 (4): 391–398. doi:10.1046/j.1468-1331.2003.00611.x. PMID 12823491. S2CID 31352244.
- Arnt J, Bøgesø KP, Hyttel J, Meier E (March 1988). "Relative dopamine D1 and D2 receptor affinity and efficacy determine whether dopamine agonists induce hyperactivity or oral stereotypy in rats". Pharmacology & Toxicology. 62 (3): 121–130. doi:10.1111/j.1600-0773.1988.tb01859.x. PMID 3259694.
- ^ Rocchi L, Chiari L, Horak FB (September 2002). "Effects of deep brain stimulation and levodopa on postural sway in Parkinson's disease". Journal of Neurology, Neurosurgery, and Psychiatry. 73 (3): 267–274. doi:10.1136/jnnp.73.3.267. PMC 1738049. PMID 12185157.
- Bloem BR, Beckley DJ, van Dijk JG, Zwinderman AH, Remler MP, Roos RA (September 1996). "Influence of dopaminergic medication on automatic postural responses and balance impairment in Parkinson's disease". Movement Disorders. 11 (5): 509–521. doi:10.1002/mds.870110506. PMID 8866492. S2CID 22509108.
- Lewis GN, Byblow WD, Walt SE (October 2000). "Stride length regulation in Parkinson's disease: the use of extrinsic, visual cues". Brain. 123 (Pt 10): 2077–2090. doi:10.1093/brain/123.10.2077. PMID 11004125.
- ^ Jiang Y, Norman KE (January 2006). "Effects of visual and auditory cues on gait initiation in people with Parkinson's disease". Clinical Rehabilitation. 20 (1): 36–45. doi:10.1191/0269215506cr925oa. PMID 16502748. S2CID 44581046.
- McCandless, P. J.; Evans, B. J.; Janssen, J.; Selfe, J.; Churchill, A.; Richards, J. (February 2016). "Effect of three cueing devices for individuals diagnosed with Parkinson's disease experiencing gait initiation challenges". Gait Posture. 44: 7–11. doi:10.1016/j.gaitpost.2015.11.006. PMC 4863931. PMID 27004625.
- McAuley JH, Daly PM, Curtis CR (August 2009). "A preliminary investigation of a novel design of visual cue glasses that aid gait in Parkinson's disease". Clinical Rehabilitation. 23 (8): 687–695. doi:10.1177/0269215509104170. PMID 19403552. S2CID 26522478.
- Parakkal Unni M, Menon PP, Livi L, Wilson MR, Young WR, Bronte-Stewart HM, Tsaneva-Atanasova K (2020). "Data-Driven Prediction of Freezing of Gait Events From Stepping Data". Frontiers in Medical Technology. 2: 581264. doi:10.3389/fmedt.2020.581264. PMC 8757792. PMID 35047881.
- Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, Silburn PA, et al. (March 2014). "Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus" (PDF). Nature Neuroscience. 17 (3): 449–454. doi:10.1038/nn.3642. PMID 24487235. S2CID 405368.
- Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain JS, et al. (May 2012). "A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation". Brain. 135 (Pt 5): 1446–1454. doi:10.1093/brain/aws039. PMC 3338924. PMID 22396391.
- ^ Davis JT, Lyons KE, Pahwa R (July 2006). "Freezing of gait after bilateral subthalamic nucleus stimulation for Parkinson's disease". Clinical Neurology and Neurosurgery. 108 (5): 461–4. doi:10.1016/j.clineuro.2005.07.008. PMID 16139421. S2CID 39409253.
- Yokoyama T, Sugiyama K, Nishizawa S, Yokota N, Ohta S, Uemura K (July 1999). "Subthalamic nucleus stimulation for gait disturbance in Parkinson's disease". Neurosurgery. 45 (1): 41–49. doi:10.1227/00006123-199907000-00011. PMID 10414565.
- Su D, Chen H, Hu W, Liu Y, Wang Z, Wang X, et al. (September 2018). "Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson's disease: a meta-analysis of controlled trials". Scientific Reports. 8 (1): 14456. Bibcode:2018NatSR...814456S. doi:10.1038/s41598-018-32161-3. PMC 6160461. PMID 30262859.
- Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM (September 2005). "Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding?". The European Journal of Neuroscience. 22 (5): 1248–56. doi:10.1111/j.1460-9568.2005.04298.x. PMID 16176368. S2CID 1961890.
- O'Shea S, Morris ME, Iansek R (September 2002). "Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks". Physical Therapy. 82 (9): 888–897. doi:10.1093/ptj/82.9.888. PMID 12201803.
- de Goede CJ, Keus SH, Kwakkel G, Wagenaar RC (April 2001). "The effects of physical therapy in Parkinson's disease: a research synthesis". Archives of Physical Medicine and Rehabilitation. 82 (4): 509–515. doi:10.1053/apmr.2001.22352. PMID 11295012.
- ^ O'Sullivan SO (2007). "Parkinson's Disease: Physical Therapy Intervention.". In O'Sullivan SB, Schmitz TJ (eds.). Physical Rehabilitation (5th ed.). Philadelphia: E.A. Davis Company. pp. 853–893.
- Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC (2005). "Gait and step training to reduce falls in Parkinson's disease". NeuroRehabilitation. 20 (3): 183–190. doi:10.3233/NRE-2005-20305. PMID 16340099.
- ^ Morris ME, Martin CL, Schenkman ML (February 2010). "Striding out with Parkinson disease: evidence-based physical therapy for gait disorders". Physical Therapy. 90 (2): 280–288. doi:10.2522/ptj.20090091. PMC 2816030. PMID 20022998.
- Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D (August 2006). "Changes in motor subtype and risk for incident dementia in Parkinson's disease". Movement Disorders. 21 (8): 1123–1130. doi:10.1002/mds.20897. PMID 16637023. S2CID 12120737.
- Burn DJ, Rowan EN, Allan LM, Molloy S, O'Brien JT, McKeith IG (May 2006). "Motor subtype and cognitive decline in Parkinson's disease, Parkinson's disease with dementia, and dementia with Lewy bodies". Journal of Neurology, Neurosurgery, and Psychiatry. 77 (5): 585–589. doi:10.1136/jnnp.2005.081711. PMC 2117449. PMID 16614017.
- Factor SA, Steenland NK, Higgins DS, Molho ES, Kay DM, Montimurro J, et al. (May 2011). "Postural instability/gait disturbance in Parkinson's disease has distinct subtypes: an exploratory analysis". Journal of Neurology, Neurosurgery, and Psychiatry. 82 (5): 564–568. doi:10.1136/jnnp.2010.222042. PMC 4646086. PMID 20884673.
External links
| Signs and symptoms relating to movement and gait | |
|---|---|
| Gait |
|
| Coordination | |
| Abnormal movement | |
| Posturing |
|
| Paralysis | |
| Weakness | |
| Range of motion | |
| Other | |