This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
Fiber photometry is a calcium imaging technique that captures 'bulk' or population-level calcium (Ca) activity from specific cell-types within a brain region or functional network in order to study neural circuits Population-level calcium activity can be correlated with behavioral tasks, such as spatial learning, memory recall and goal-directed behaviors. The technique involves the surgical implantation of fiber optics into the brains of living animals. The benefits to researchers are that optical fibers are simpler to implant, less invasive and less expensive than other calcium methods, and there is less weight and stress on the animal, as compared to miniscopes. It also allows for imaging of multiple interacting brain regions and integration with other neuroscience techniques. The limitations of fiber photometry are low cellular and spatial resolution, and the fact that animals must be securely tethered to a rigid fiber bundle, which may impact the naturalistic behavior of smaller mammals such as mice.
Technical description
Fiber photometry relies on the expression of genetically encoded calcium indicators (GECIs), like GCaMP or RCaMP, which can be targeted to specific cells using cell-specific promoters like Ca2+/calmodulin-dependent protein kinase II (CaMKII) and human synapsin (hSyn) that confer excitatory neuronal and pan-neuronal expression, respectively. These promoters can be used to target various neuronal subtypes as well as non-neuronal cells that exhibit calcium dynamics, such as astrocytes, using the glial fibrillary acidic protein (GFAP) promoter. In both neurons and astrocytes, cellular activity in the form of action potentials, exocytosis of neurotransmitters, changes in synaptic plasticity and gene transcription is coupled to an influx of Ca ions.
These activity-dependent changes in intracellular calcium levels can be monitored by introducing GECIs to the cell. Following this influx of ions, GECIs fluoresce upon Ca binding and the change in fluorescence corresponds proportionally to intracellular calcium changes. The most commonly used calcium indicator for fiber photometry (and other in vivo imaging method) is GCaMP6, although additional GECIs continue to be developed with unique fluorescence spectra, kinetics, signal-to-noise ratios and calcium-sensitivities. These indicators can be expressed in the brain in two main ways: viral expression and transgenic mouse lines. Recently, there has been a growing list of indicators that have become available to measure different chemical signals, like dLight to record dopamine signaling, or OxLight to record orexin, for example. GCaMP, RCaMP, dLight and other indicators are excited by a light source at an optimal wavelength and emit their own light in return, allowing for recording of calcium or neurotransmitter dynamics across time.
Fiber photometry systems are designed to deliver precise excitation wavelengths of light that are specific to a calcium (e.g. GCaMP) or neurotransmitter indicator (e.g. dLight). This light travels down an optical fiber to a fiber optic that is implanted in the brain region or regions of interest. The calcium indicator is that is expressed in a cell-type specific manner is excited by this light and in turn, emits its own signal that travels back through the same fiber. These collected emission signals are spectrally-separated by a dichroic mirror, passed through a filter and focused onto a photodetector, scientific camera, or PMT. The collected signal represents a change in fluorescence (ΔF) relative to an initial baseline (F). In turn, researchers can observe a signal that corresponds to calcium transients (ΔF/F). This time series data can be analyzed using a variety of open-source pipelines, such as pMAT, pyPhotometry and GuPPy.
Importantly, isosbestic signals are calcium-independent signals (i.e. approximately 405-415 nm) that are in contrast to the calcium-dependent wavelength (i.e. 470 nm for GCaMP). Because GCaMP has two states, Ca bound and un-bound, the two protein conformations have unique excitation and emission spectra. The wavelength of excitation at which GCaMP has the same absorbance with and without Ca is the isosbestic and determines the calcium-independent fluorescence baseline. Animal motion and tissue autofluorescence are reflected in this calcium-independent signal and can be subtracted or regressed to reveal the true change in fluorescence.
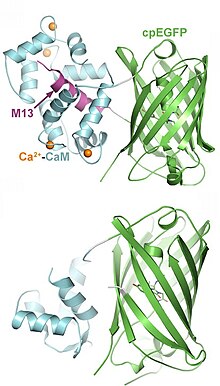
Genetically-encoded calcium indicators (GECIs)
Expression
Optimal expression of genetically encoded calcium indicators (GECIs) can be accomplished in two ways: adeno-associated viral vectors and transgenic rodent lines. Viral injection requires GECI infusion into the brain region of interest. This virus can be targeted to infect unique cell-types through the use of cell-specific promoters like CaMKII and GFAP to target excitatory neurons and astrocytes, respectively. This allows for neural activity to be recorded from a genetically defined subpopulation of neurons or glial cells through an implanted optical fiber. Viral expression requires titration of dilutions to obtain optimal expression in the brain, which may be necessary instead of using transgenic rodent lines for certain experiments. Expression of GECIs can also be accomplished through the use of transgenic lines that allow for ubiquitous expression throughout the entire brain. Depending on the strain of mouse or rat, the expression can vary across brain regions, posing potential challenges during recording.
GCaMP
GCaMP is a genetically encoded calcium indicator (GECI) that is commonly used in multiple imaging methods. GCaMP emits fluorescence when the indicator is bound to a calcium ion (Ca). This calcium signal is directly tied to neural response patterns, neurotransmitter release, and membrane excitability. The excitation wavelengths for GCaMP and its isosbestic signal are approximately 470 nm and 415 nm (blue), respectively. The goal is to photo-excite the maximum absorption and isosbestic points of the indicator. The isosbestic point of GCaMP is the calcium-independent signal, approximately 405-415 nm. This is determined based on GCaMP having two functional states, bound and unbound to calcium ions. These two states have unique protein excitation and emission spectra. The wavelength of excitation where these two protein states have the same absorbance is the isosbestic point and determines the baseline fluorescence. Motion and autofluorescence are reflected in this calcium-independent signal and can be subtracted or regressed to reveal the true change in cellular fluorescence during a behavioral task or experimental manipulation.
The emission wavelength of GCaMP is approximately 525 nm (green), which can be analyzed and correlated across time during behavioral tasks.
Multiple-color fiber photometry
To observe simultaneous calcium dynamics in multiple cell types, researchers have combined two or more GECIs in a single brain region. For example, a research group recorded fluorescence from the green and red GECIs, GCaMP6f and jRGECO1a, that were differentially expressed in striatal direct- and indirect-pathway spiny projection neurons in freely behaving mice. The expression of multiple GECIs in the same brain region can not only be performed in two sets of neurons, as shown in the previous study. These simultaneous recordings of bulk calcium can also be performed with multiple cell types, such as neurons and astrocytes. These cell types express unique promoters, such as GFAP and hSyn, and the GECIs can be targeted specifically in this way. Another research group performed successful dual-color fiber photometry using astrocyte- and neuron-specific promoters while mice freely performed a working memory task (T-maze).
Equipment
The goal of fiber photometry is to precisely deliver, extract and record bulk calcium signal from specific populations of cells within a brain region of interest. To access the signal, an optical cannula/fiber must be surgically implanted at the site of GECI expression. This optical fiber can only collect population-level calcium signal, not individual cell activity. Additionally, optical fibers allow recording from both deep and shallow brain structures, and minimize tissue damage unlike GRIN lenses or cortical windows.
GCaMP has specific excitation and emission spectra at approximately 470 nm and 530 nm, respectively. LED light sources are amongst the most commonly selected, due to the low optical power necessary to excite GECIs. Proper transmission of the excitation and emission signals bidirectionally from the brain to the imaging device is coordinated by dichroic mirrors and excitation/emission filters. There are three different types of imaging devices: photodetectors, photomultiplier tubes (PMTs) and scientific cameras. When collecting signal from a single brain region, it is typical to use a photodetector or PMT due to their fast acquisition and low signal-to-noise ratio (SNR). Alternatively, when collecting from multiple brain regions, scientific cameras or multiple photodetectors or PMTs must be used.
Overall, this system allows for coupling between the optical cannula, light source and imaging device. Precise light delivery to the GECI is enabled by the optical cannula/fiber and the emission signal is collected by the imaging device during recording.
Some examples of commercially available fiber photometry systems include Neurophotometrics, Inscopix and MightexBio.
Benefits and limitations
All scientific methods have considerations that must be taken into account before use. Fiber photometry has many benefits over other techniques for calcium imaging, but it comes with limitations.
Benefits
For individuals in labs that want to integrate calcium imaging into their experiments but may not have the financial or technical circumstances to do so yet, fiber photometry is a low barrier of entry. Optical fibers are simpler to implant, less invasive and are more inexpensive than other calcium methods such as one- or two-photon imaging. It is good for longitudinal behavioral paradigms because there is less weight and stress on the animal, as compared to miniscopes. It limits mobility of the animal significantly less than other methods, allowing for more freely-moving, naturalistic behaviors in larger rodent models. It is a versatile technique, allowing for imaging of multiple interacting brain regions and integration with optogenetics, electrophysiology and more systems-level neuroscience techniques. More recently, this technique can be coupled with other fluorescent indicators for neurotransmitter activity or pH changes.
Limitations
It is important when planning experiments to consider the major limitation of fiber photometry: low cellular and spatial resolution. This lack of optical resolution can be attributed to the collection of an aggregation of activity within a field of view, only allowing for 'bulk' changes in fluorescent signal. Although the size of an optical cannula is much smaller than technology used in other calcium imaging methods, such as one- and two-photon microscopy, animals must be securely tethered to a rigid fiber bundle. This may limit the naturalistic behavior and of smaller mammals, such as mice.
Integration with other methods

Optogenetics and DREADDs
Fiber photometry can be integrated with cellular manipulation to draw a causal link between neural activity and behavior. Targeted modulation of defined cell types and projections in the brain can be accomplished using optogenetics or Designer Receptors Exclusively Activated by Designer Drugs (DREADDs). The method is chosen based on the temporal precision necessary for the experimental design, amongst other factors. Optogenetics allows for manipulation of a specific cell-type with high temporal precision. DREADDs have a much lower temporal precision due to the pharmacokinetics of the ligand, such as clozapine-N-oxide (CNO) or deschloroclozapine (DCZ). It is important to note that simultaneous optical readout and optogenetic manipulation comes with several potential issues that are discussed below.
In vivo electrophysiology
Additionally, fiber photometry can be coupled with in vivo electrophysiology within the same animal. When combined, this combination of electrophysiological recording and calcium imaging can be used to observe cell-type specific activity with higher temporal precision read-outs of neuronal action potentials in freely-behaving animal models.
Potential problems and solutions
Delivery and expression of optogenetic probes and calcium indicators to the same neurons can pose problems. Calcium indicators and manipulation channels can have overlapping excitation spectrums, such as GCaMP and channelrhodopsin (ChR2), which both have a peak wavelength of excitation at approximately 470 nm. Excitation of the calcium indicator can potentially activate the optogenetic light-sensitive ion channel. The measured change in calcium signal cannot be easily attributed to actual changes in calcium or optogenetic-induced signal. Solutions to this issue include the combination of indicators that have non-overlapping excitation spectrum with your optogenetic probe or calcium indicator. Calcium indicators (GCaMP, RCaMP) and optogenetic probes for excitation (ChR2, Crimson) and inhibition (eNpHR, Arch, Jaws) are options for this.
Other calcium imaging methods
Miniscopes and single-photon imaging
Miniscopes are head-mounted, miniature microscopes that allow imaging of large populations of neural activity in freely-behaving mice and rats. This is possible due to their small size, as they are light enough for a mouse or rat to easily carry without interfering greatly with behavior. Researchers couple miniscopes with implanted gradient-refractive-index (GRIN) lenses or cortical windows that enable deep and superficial brain imaging. This method is ideal for monitoring the activity of hundreds of genetically- and spatially-defined cells within a single animal. As compared to fiber photometry, miniscopes allow imaging with high cellular resolution, detecting changes in calcium within individual neurons and non-neuronal cells. Additionally, this method enables repeated imaging over time to analyze the transition from 'healthy' to pathological states or changes over the course of behavior. However, this method of imaging has low sensitivity and high noise, producing lower resolution imaging compared to other multi-photon methods like two-photon. These miniature microscopes are limited in their ability to detect far-red-shifted indicators that would be necessary for combination of optogenetics and calcium imaging here, as is discussed above.
Two-photon imaging
Two-photon imaging is another calcium imaging method that records fluctuations in cellular GECI dynamics. It provides a way to penetrate highly light-scattering brain tissue up to 600-700 microns below the surface of the brain. As compared to other techniques, two-photon offers higher cellular and sub-cellular spatial resolution, such as within dendrites and axonal boutons, within a well-defined focal plane. However, without the assistance of an optical cannula or micro-endoscope, this method is limited to more superficial brain areas. This type of imaging also requires that the animals remain head-fixed, limiting naturalistic behaviors necessary for some complex behavioral tasks.
References
- ^ Cui, Guohong; Jun, Sang Beom; Jin, Xin; Luo, Guoxiang; Pham, Michael D.; Lovinger, David M.; Vogel, Steven S.; Costa, Rui M. (June 2014). "Deep brain optical measurements of cell type–specific neural activity in behaving mice". Nature Protocols. 9 (6): 1213–1228. doi:10.1038/nprot.2014.080. ISSN 1750-2799. PMC 4100551. PMID 24784819.
- Kim, Christina K.; Yang, Samuel J.; Pichamoorthy, Nandini; Young, Noah P.; Kauvar, Isaac; Jennings, Joshua H.; Lerner, Talia N.; Berndt, Andre; Lee, Soo Yeun; Ramakrishnan, Charu; Davidson, Thomas J. (April 2016). "Simultaneous fast measurement of circuit dynamics at multiple sites across the mammalian brain". Nature Methods. 13 (4): 325–328. doi:10.1038/nmeth.3770. ISSN 1548-7105. PMC 5717315. PMID 26878381.
- ^ Wang, Yuanmo; DeMarco, Emily M.; Witzel, Lisa Sophia; Keighron, Jacqueline D. (2021-02-01). "A selected review of recent advances in the study of neuronal circuits using fiber photometry". Pharmacology Biochemistry and Behavior. 201: 173113. doi:10.1016/j.pbb.2021.173113. ISSN 0091-3057. PMID 33444597. S2CID 231579597.
- ^ Gunaydin, Lisa A.; Grosenick, Logan; Finkelstein, Joel C.; Kauvar, Isaac V.; Fenno, Lief E.; Adhikari, Avishek; Lammel, Stephan; Mirzabekov, Julie J.; Airan, Raag D.; Zalocusky, Kelly A.; Tye, Kay M. (2014-06-19). "Natural neural projection dynamics underlying social behavior". Cell. 157 (7): 1535–1551. doi:10.1016/j.cell.2014.05.017. ISSN 1097-4172. PMC 4123133. PMID 24949967.
- ^ Resendez, Shanna L; Stuber, Garret D (January 2015). "In vivo Calcium Imaging to Illuminate Neurocircuit Activity Dynamics Underlying Naturalistic Behavior". Neuropsychopharmacology. 40 (1): 238–239. doi:10.1038/npp.2014.206. ISSN 0893-133X. PMC 4262901. PMID 25482169.
- Sych, Yaroslav; Chernysheva, Maria; Sumanovski, Lazar T.; Helmchen, Fritjof (June 2019). "High-density multi-fiber photometry for studying large-scale brain circuit dynamics". Nature Methods. 16 (6): 553–560. doi:10.1038/s41592-019-0400-4. ISSN 1548-7105. PMID 31086339. S2CID 152283372.
- ^ Girven, Kasey S.; Sparta, Dennis R. (2017-02-15). "Probing Deep Brain Circuitry: New Advances in in Vivo Calcium Measurement Strategies". ACS Chemical Neuroscience. 8 (2): 243–251. doi:10.1021/acschemneuro.6b00307. PMID 27984692.
- Nimmerjahn, Axel; Bergles, Dwight E (2015-06-01). "Large-scale recording of astrocyte activity". Current Opinion in Neurobiology. Large-Scale Recording Technology (32). 32: 95–106. doi:10.1016/j.conb.2015.01.015. ISSN 0959-4388. PMC 4447573. PMID 25665733.
- Tsunematsu, Tomomi; Sakata, Shuzo; Sanagi, Tomomi; Tanaka, Kenji F.; Matsui, Ko (2021-06-23). "Region-Specific and State-Dependent Astrocyte Ca2+ Dynamics during the Sleep-Wake Cycle in Mice". Journal of Neuroscience. 41 (25): 5440–5452. doi:10.1523/JNEUROSCI.2912-20.2021. ISSN 0270-6474. PMC 8221592. PMID 34006590.
- Paukert, Martin; Agarwal, Amit; Cha, Jaepyeong; Doze, Van A.; Kang, Jin U.; Bergles, Dwight E. (2014-06-18). "Norepinephrine controls astroglial responsiveness to local circuit activity". Neuron. 82 (6): 1263–1270. doi:10.1016/j.neuron.2014.04.038. ISSN 1097-4199. PMC 4080721. PMID 24945771.
- Corkrum, Michelle; Araque, Alfonso (October 2021). "Astrocyte-neuron signaling in the mesolimbic dopamine system: the hidden stars of dopamine signaling". Neuropsychopharmacology. 46 (11): 1864–1872. doi:10.1038/s41386-021-01090-7. ISSN 1740-634X. PMC 8429665. PMID 34253855.
- Wang, Wenjing; Kim, Christina K.; Ting, Alice Y. (February 2019). "Molecular tools for imaging and recording neuronal activity". Nature Chemical Biology. 15 (2): 101–110. doi:10.1038/s41589-018-0207-0. ISSN 1552-4469. PMID 30659298. S2CID 58607817.
- ^ Chen, Tsai-Wen; Wardill, Trevor J.; Sun, Yi; Pulver, Stefan R.; Renninger, Sabine L.; Baohan, Amy; Schreiter, Eric R.; Kerr, Rex A.; Orger, Michael B.; Jayaraman, Vivek; Looger, Loren L. (July 2013). "Ultrasensitive fluorescent proteins for imaging neuronal activity". Nature. 499 (7458): 295–300. Bibcode:2013Natur.499..295C. doi:10.1038/nature12354. ISSN 1476-4687. PMC 3777791. PMID 23868258.
- ^ Vickstrom, Casey R.; Snarrenberg, Shana Terai; Friedman, Vladislav; Liu, Qing-song (2021-06-18). "Application of optogenetics and in vivo imaging approaches for elucidating the neurobiology of addiction". Molecular Psychiatry. 27 (1): 640–651. doi:10.1038/s41380-021-01181-3. ISSN 1476-5578. PMC 9190069. PMID 34145393. S2CID 235466802.
- ^ Leopold, Anna V.; Shcherbakova, Daria M.; Verkhusha, Vladislav V. (2019). "Fluorescent Biosensors for Neurotransmission and Neuromodulation: Engineering and Applications". Frontiers in Cellular Neuroscience. 13: 474. doi:10.3389/fncel.2019.00474. ISSN 1662-5102. PMC 6819510. PMID 31708747.
- ^ Dana, Hod; Chen, Tsai-Wen; Hu, Amy; Shields, Brenda C.; Guo, Caiying; Looger, Loren L.; Kim, Douglas S.; Svoboda, Karel (2014-09-24). "Thy1-GCaMP6 Transgenic Mice for Neuronal Population Imaging In Vivo". PLOS ONE. 9 (9): e108697. Bibcode:2014PLoSO...9j8697D. doi:10.1371/journal.pone.0108697. ISSN 1932-6203. PMC 4177405. PMID 25250714.
- Cosme, Caitlin V.; Palissery, Gates K.; Lerner, Talia N. (2018-09-01). "A dLight-ful New View of Neuromodulation". Trends in Neurosciences. 41 (9): 566–568. doi:10.1016/j.tins.2018.07.004. ISSN 0166-2236. PMC 6519934. PMID 30055832.
- Duffet, Loïc; Kosar, Seher; Panniello, Mariangela; Viberti, Bianca; Bracey, Edward; Zych, Anna D.; Radoux-Mergault, Arthur; Zhou, Xuehan; Dernic, Jan; Ravotto, Luca; Tsai, Yuan-Chen (February 2022). "A genetically encoded sensor for in vivo imaging of orexin neuropeptides". Nature Methods. 19 (2): 231–241. doi:10.1038/s41592-021-01390-2. ISSN 1548-7105. PMC 8831244. PMID 35145320.
- ^ Tian, Lin; Hires, S. Andrew; Mao, Tianyi; Huber, Daniel; Chiappe, M. Eugenia; Chalasani, Sreekanth H.; Petreanu, Leopoldo; Akerboom, Jasper; McKinney, Sean A.; Schreiter, Eric R.; Bargmann, Cornelia I. (December 2009). "Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators". Nature Methods. 6 (12): 875–881. doi:10.1038/nmeth.1398. ISSN 1548-7105. PMC 2858873. PMID 19898485.
- Inoue, Masatoshi; Takeuchi, Atsuya; Horigane, Shin-ichiro; Ohkura, Masamichi; Gengyo-Ando, Keiko; Fujii, Hajime; Kamijo, Satoshi; Takemoto-Kimura, Sayaka; Kano, Masanobu; Nakai, Junichi; Kitamura, Kazuo (January 2015). "Rational design of a high-affinity, fast, red calcium indicator R-CaMP2". Nature Methods. 12 (1): 64–70. doi:10.1038/nmeth.3185. ISSN 1548-7105. PMID 25419959. S2CID 52798973.
- ^ Patriarchi, Tommaso; Cho, Jounhong Ryan; Merten, Katharina; Howe, Mark W.; Marley, Aaron; Xiong, Wei-Hong; Folk, Robert W.; Broussard, Gerard Joey; Liang, Ruqiang; Jang, Min Jee; Zhong, Haining (2018-06-29). "Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors". Science. 360 (6396): eaat4422. doi:10.1126/science.aat4422. ISSN 0036-8075. PMC 6287765. PMID 29853555.
- Martianova, Ekaterina; Aronson, Sage; Proulx, Christophe D. (2019-10-20). "Multi-Fiber Photometry to Record Neural Activity in Freely-Moving Animals". Journal of Visualized Experiments (152): e60278. doi:10.3791/60278. ISSN 1940-087X. PMID 31680685. S2CID 207898488.
- Bruno, Carissa A.; O'Brien, Chris; Bryant, Svetlana; Mejaes, Jennifer I.; Estrin, David J.; Pizzano, Carina; Barker, David J. (February 2021). "pMAT: An open-source software suite for the analysis of fiber photometry data". Pharmacology, Biochemistry, and Behavior. 201: 173093. doi:10.1016/j.pbb.2020.173093. ISSN 1873-5177. PMC 7853640. PMID 33385438.
- Akam, Thomas; Walton, Mark E. (2019-03-05). "pyPhotometry: Open source Python based hardware and software for fiber photometry data acquisition". Scientific Reports. 9 (1): 3521. Bibcode:2019NatSR...9.3521A. doi:10.1038/s41598-019-39724-y. ISSN 2045-2322. PMC 6401057. PMID 30837543.
- Sherathiya, Venus N.; Schaid, Michael D.; Seiler, Jillian L.; Lopez, Gabriela C.; Lerner, Talia N. (2021-07-16). "GuPPy, a Python toolbox for the analysis of fiber photometry data". Scientific Reports. 11 (1): 2021.07.15.452555. Bibcode:2021NatSR..1124212S. doi:10.1038/s41598-021-03626-9. PMC 8688475. PMID 34930955.
- ^ Siciliano, Cody A.; Tye, Kay M. (2019-02-01). "Leveraging calcium imaging to illuminate circuit dysfunction in addiction". Alcohol. New Technologies in Alcohol Research and Treatment. 74: 47–63. doi:10.1016/j.alcohol.2018.05.013. ISSN 0741-8329. PMC 7575247. PMID 30470589.
- ^ Grienberger, Christine; Konnerth, Arthur (2012-03-08). "Imaging calcium in neurons". Neuron. 73 (5): 862–885. doi:10.1016/j.neuron.2012.02.011. ISSN 1097-4199. PMID 22405199. S2CID 3023809.
- Inoue, Masatoshi (2021-08-01). "Genetically encoded calcium indicators to probe complex brain circuit dynamics in vivo". Neuroscience Research. 169: 2–8. doi:10.1016/j.neures.2020.05.013. ISSN 0168-0102. PMID 32531233. S2CID 219559849.
- Meng, Chengbo; Zhou, Jingheng; Papaneri, Amy; Peddada, Teja; Xu, Karen; Cui, Guohong (2018-05-16). "Spectrally Resolved Fiber Photometry for Multi-component Analysis of Brain Circuits". Neuron. 98 (4): 707–717.e4. doi:10.1016/j.neuron.2018.04.012. ISSN 0896-6273. PMC 5957785. PMID 29731250.
- Lin, Zhu; You, Feng; Li, Ting; Feng, Yijia; Zhao, Xinyue; Yang, Jingjing; Yao, Zhimo; Gao, Ying; Chen, Jiang-Fan (2021-10-26). "Entrainment of Astrocytic and Neuronal Ca2+ Population Dynamics During Information Processing of Working Memory in Mice". Neuroscience Bulletin. 38 (5): 474–488. doi:10.1007/s12264-021-00782-w. ISSN 1995-8218. PMC 9106780. PMID 34699030. S2CID 239888986.
- Li, Yi; Liu, Zhixiang; Guo, Qingchun; Luo, Minmin (2019-06-01). "Long-term Fiber Photometry for Neuroscience Studies". Neuroscience Bulletin. 35 (3): 425–433. doi:10.1007/s12264-019-00379-4. ISSN 1995-8218. PMC 6527730. PMID 31062336.
- ^ Emiliani, Valentina; Cohen, Adam E.; Deisseroth, Karl; Häusser, Michael (2015-10-14). "All-Optical Interrogation of Neural Circuits". Journal of Neuroscience. 35 (41): 13917–13926. doi:10.1523/JNEUROSCI.2916-15.2015. ISSN 0270-6474. PMC 4604230. PMID 26468193.
- ^ Patel, Amisha A.; McAlinden, Niall; Mathieson, Keith; Sakata, Shuzo (2020). "Simultaneous Electrophysiology and Fiber Photometry in Freely Behaving Mice". Frontiers in Neuroscience. 14: 148. doi:10.3389/fnins.2020.00148. ISSN 1662-453X. PMC 7047771. PMID 32153363.
- Armbruster, Blaine N.; Li, Xiang; Pausch, Mark H.; Herlitze, Stefan; Roth, Bryan L. (2007-03-20). "Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand". Proceedings of the National Academy of Sciences of the United States of America. 104 (12): 5163–5168. doi:10.1073/pnas.0700293104. ISSN 0027-8424. PMC 1829280. PMID 17360345.
- Nagai, Yuji; Miyakawa, Naohisa; Takuwa, Hiroyuki; Hori, Yukiko; Oyama, Kei; Ji, Bin; Takahashi, Manami; Huang, Xi-Ping; Slocum, Samuel T.; DiBerto, Jeffrey F.; Xiong, Yan (September 2020). "Deschloroclozapine, a potent and selective chemogenetic actuator enables rapid neuronal and behavioral modulations in mice and monkeys". Nature Neuroscience. 23 (9): 1157–1167. doi:10.1038/s41593-020-0661-3. ISSN 1546-1726. PMID 32632286. S2CID 220375204.
- ^ Resendez, Shanna L.; Jennings, Josh H.; Ung, Randall L.; Namboodiri, Vijay Mohan K.; Zhou, Zhe Charles; Otis, James M.; Nomura, Hiroshi; McHenry, Jenna A.; Kosyk, Oksana; Stuber, Garret D. (March 2016). "Visualization of cortical, subcortical and deep brain neural circuit dynamics during naturalistic mammalian behavior with head-mounted microscopes and chronically implanted lenses". Nature Protocols. 11 (3): 566–597. doi:10.1038/nprot.2016.021. ISSN 1750-2799. PMC 5239057. PMID 26914316.
- ^ Ziv, Yaniv; Burns, Laurie D.; Cocker, Eric D.; Hamel, Elizabeth O.; Ghosh, Kunal K.; Kitch, Lacey J.; El Gamal, Abbas; Schnitzer, Mark J. (March 2013). "Long-term dynamics of CA1 hippocampal place codes". Nature Neuroscience. 16 (3): 264–266. doi:10.1038/nn.3329. ISSN 1097-6256. PMC 3784308. PMID 23396101.
- ^ Ghosh, Kunal K.; Burns, Laurie D.; Cocker, Eric D.; Nimmerjahn, Axel; Ziv, Yaniv; Gamal, Abbas El; Schnitzer, Mark J. (October 2011). "Miniaturized integration of a fluorescence microscope". Nature Methods. 8 (10): 871–878. doi:10.1038/nmeth.1694. ISSN 1548-7105. PMC 3810311. PMID 21909102.
- Jung, Juergen C.; Mehta, Amit D.; Aksay, Emre; Stepnoski, Raymond; Schnitzer, Mark J. (2004-11-01). "In Vivo Mammalian Brain Imaging Using One- and Two-Photon Fluorescence Microendoscopy". Journal of Neurophysiology. 92 (5): 3121–3133. doi:10.1152/jn.00234.2004. ISSN 0022-3077. PMC 2826362. PMID 15128753.
- Levene, Michael J.; Dombeck, Daniel A.; Kasischke, Karl A.; Molloy, Raymond P.; Webb, Watt W. (2004-04-01). "In Vivo Multiphoton Microscopy of Deep Brain Tissue". Journal of Neurophysiology. 91 (4): 1908–1912. doi:10.1152/jn.01007.2003. ISSN 0022-3077. PMID 14668300.
- ^ Svoboda, Karel; Yasuda, Ryohei (2006-06-15). "Principles of two-photon excitation microscopy and its applications to neuroscience". Neuron. 50 (6): 823–839. doi:10.1016/j.neuron.2006.05.019. ISSN 0896-6273. PMID 16772166. S2CID 7278880.
- Denk, W.; Delaney, K. R.; Gelperin, A.; Kleinfeld, D.; Strowbridge, B. W.; Tank, D. W.; Yuste, R. (1994-10-01). "Anatomical and functional imaging of neurons using 2-photon laser scanning microscopy". Journal of Neuroscience Methods. Imaging Techniques in Neurobiology. 54 (2): 151–162. doi:10.1016/0165-0270(94)90189-9. ISSN 0165-0270. PMID 7869748. S2CID 3772937.
- Dombeck, Daniel; Tank, David (2014-07-01). "Two-Photon Imaging of Neural Activity in Awake Mobile Mice". Cold Spring Harbor Protocols. 2014 (7): 726–736. doi:10.1101/pdb.top081810. ISSN 1940-3402. PMID 24987148.
- Dombeck, Daniel A.; Khabbaz, Anton N.; Collman, Forrest; Adelman, Thomas L.; Tank, David W. (2007-10-04). "Imaging Large-Scale Neural Activity with Cellular Resolution in Awake, Mobile Mice". Neuron. 56 (1): 43–57. doi:10.1016/j.neuron.2007.08.003. ISSN 0896-6273. PMC 2268027. PMID 17920014.