
| |
| Names | |
|---|---|
| IUPAC name 2-Butanoyl-4--2,4,6-trihydroxyphenyl]methyl]-3,5-dihydroxy-6,6-dimethylcyclohexa-2,4-dien-1-one | |
| Other names Filixic acid BBB; Filixic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.022.516 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C36H44O12 |
| Molar mass | 668.736 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Filicin is a chemical compound that has been isolated from ferns of the genus Dryopteris. It has been isolated from the male fern (Dryopteris filix-mas). Filicin has been studied for its anthelmintic activity.
Related compounds
A variety of chemically related compounds, sometimes referred to collectively as filicins, have also been isolated from ferns. Chemical analysis of filicins in fern extracts can assist in determining taxonomy. Examples of filicins include:
| Name(s) | Chemical structure | Molecular formula | Molecular weight (g/mol) | CAS number | PubChem | Notes and references |
|---|---|---|---|---|---|---|
| Filixic acid ABA | 
|
C32H36O12 | 612.63 | 38226-84-5 | CID 15081408 from PubChem | First isolated from Dryopteris dickinsii |
| Filixic acid ABP | 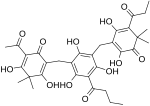
|
C33H38O12 | 626.66 | 57765-54-5 | CID 73672225 from PubChem | |
| Filixic acid ABB | 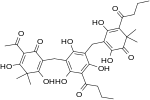
|
C34H40O12 | 640.68 | 37318-24-4 | CID 102574620 from PubChem | Found in Dryopteris sieboldii |
| Filixic acid PBP | 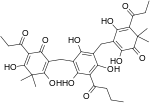
|
C34H40O12 | 640.68 | 51005-85-7 | CID 20055092 from PubChem | Found in Dryopteris sieboldii and Dryopteris filix-mas |
| Filixic acid PBB | 
|
C35H42O12 | 654.71 | 49582-09-4 | CID 20055091 from PubChem | |
| Flavaspidic acid BB; Toxifren; Polystichocitrin; Glavaspidic acid | 
|
C24H30O8 | 446.49 | 114-42-1 | CID 8237 from PubChem | Isolated from Dryopteris abbreviata |
References
- "Filicin". TOXNET. U.S. National Library of Medicine.
- Heikinheimo, R (1963). "Effect of filicin administered as an anthelmintic on the coagulation factors of the blood". Annales Medicinae Internae Fenniae. 52: 93–6. PMID 13953368.
- Euw, J. v.; Lounasmaa, M.; Reichstein, T.; Widén, C.J. (1980). "Chemotaxonomy in Dryopteris and related fern genera". Studia Geobotanica. 1: 275–311.
- Hisada, Sueo; Shiraishi, Koichi; Inagaki, Isao (1972). "Pharmaceutical studies on Japanese ferns containing phloroglucinol derivatives. 9 Constituents of Dryopteris dickinsii". Yakugaku Zasshi. 92 (9): 1124–1128. doi:10.1248/yakushi1947.92.9_1124. PMID 4674828.
- ^ Widén, C. J; Lounasmaa, M; Sarvela, J (1975). "Phloroglucinol derivatives of eleven Dryopteris species from Japan". Planta Medica. 28 (2): 144–64. doi:10.1055/s-0028-1097844. PMID 1197418.
- ^ Hisada, Sueo; Inoue, O.; Inagak, Isao (1973). "Phloroglucinol derivatives of Dryopteris sieboldii". Phytochemistry. 12 (8): 2055. doi:10.1016/s0031-9422(00)91535-8.
- Coşkun, Maksut; Sakushima, Akiyo; Nishibe, Sansei; Hisada, Sueo; Tanker, Nevin (1982). "A phloroglucinol derivative of Dryopteris abbreviata". Phytochemistry. 21 (6): 1453. doi:10.1016/0031-9422(82)80168-4.