Pharmaceutical compound
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈfoʊlɪk, ˈfɒlɪk/ |
| Trade names | Folicet, Folvite |
| Other names | Wills factor, FA, N-(4-{amino}benzoyl)-L-glutamic acid, pteroyl-L-glutamic acid, folacin, vitamin B9; formerly, vitamin Bc and vitamin M |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682591 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular, intravenous, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50–100% |
| Metabolism | Liver |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.381 |
| Chemical and physical data | |
| Formula | C19H19N7O6 |
| Molar mass | 441.404 g·mol |
| 3D model (JSmol) | |
| Density | 1.6±0.1 g/cm |
| Melting point | 250 °C (482 °F) (decomposition) |
| Solubility in water | 1.6mg/L (25 °C) |
SMILES
| |
InChI
| |
Folate, also known as vitamin B9 and folacin, is one of the B vitamins. Manufactured folic acid, which is converted into folate by the body, is used as a dietary supplement and in food fortification as it is more stable during processing and storage. Folate is required for the body to make DNA and RNA and metabolise amino acids necessary for cell division and maturation of blood cells. As the human body cannot make folate, it is required in the diet, making it an essential nutrient. It occurs naturally in many foods. The recommended adult daily intake of folate in the U.S. is 400 micrograms from foods or dietary supplements.
Folate in the form of folic acid is used to treat anemia caused by folate deficiency. Folic acid is also used as a supplement by women during pregnancy to reduce the risk of neural tube defects (NTDs) in the baby. NTDs include anencephaly and spina bifida, among other defects. Low levels in early pregnancy are believed to be the cause of more than half of babies born with NTDs. More than 80 countries use either mandatory or voluntary fortification of certain foods with folic acid as a measure to decrease the rate of NTDs. Long-term supplementation with relatively large amounts of folic acid is associated with a small reduction in the risk of stroke and an increased risk of prostate cancer. There are concerns that large amounts of supplemental folic acid can hide vitamin B12 deficiency.
Not consuming enough folate can lead to folate deficiency. This may result in a type of anemia in which red blood cells become abnormally large. Symptoms may include feeling tired, heart palpitations, shortness of breath, open sores on the tongue, and changes in the color of the skin or hair. Folate deficiency in children may develop within a month of poor dietary intake. In adults, normal total body folate is between 10 and 30 mg with about half of this amount stored in the liver and the remainder in blood and body tissues. In plasma, the natural folate range is 150 to 450 nM.
Folate was discovered between 1931 and 1943. It is on the World Health Organization's List of Essential Medicines. In 2022, it was the 65th most commonly prescribed medication in the United States, with more than 10 million prescriptions. The term "folic" is from the Latin word folium (which means leaf) because it was found in dark-green leafy vegetables.
Definition
Folate (vitamin B9) refers to the many forms of folic acid and its related compounds, including tetrahydrofolic acid (the active form), methyltetrahydrofolate (the primary form found in blood), methenyltetrahydrofolate, folinic acid, folacin, and pteroylglutamic acid. Historic names included L. casei factor, vitamin Bc and vitamin M.
The terms folate and folic acid have somewhat different meanings in different contexts, although sometimes used interchangeably. Within the field of organic chemistry, folate refers to the conjugate base of folic acid. Within the field of biochemistry, folates refer to a class of biologically active compounds related to and including folic acid. Within the field of nutrition, the folates are a family of essential nutrients related to folic acid obtained from natural sources whereas the term folic acid is reserved for the manufactured form that is used as a dietary supplement.
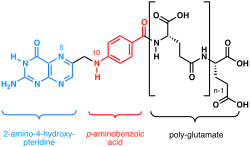
Chemically, folates consist of three distinct chemical moieties linked together. A pterin (2-amino-4-hydroxy-pteridine) heterocyclic ring is linked by a methylene bridge to a p-aminobenzoyl group that in turn is bonded through an amide linkage to either glutamic acid or poly-glutamate. One-carbon units in a variety of oxidation states may be attached to the N5 nitrogen atom of the pteridine ring and/or the N10 nitrogen atom of the p-aminobenzoyl group.
Health effects
Folate is especially important during periods of frequent cell division and growth, such as infancy and pregnancy. Folate deficiency hinders DNA synthesis and cell division, affecting hematopoietic cells and neoplasms the most because of their greater frequency of cell division. RNA transcription and subsequent protein synthesis are less affected by folate deficiency, as the mRNA can be recycled and used again (as opposed to DNA synthesis, where a new genomic copy must be created).
Birth defects
Deficiency of folate in pregnant women has been implicated in neural tube defects (NTDs), with an estimate of 300,000 cases worldwide prior to the implementation in many countries of mandatory food fortification. NTDs occur early in pregnancy (first month), therefore women must have abundant folate upon conception and for this reason there is a recommendation that any woman planning to become pregnant consume a folate-containing dietary supplement before and during pregnancy. The Center for Disease Control and Prevention (CDC) recommends a daily amount of 400 micrograms of folic acid for the prevention of NTDs. Many women take this medication less than the CDC recommends, especially in cases where the pregnancy was unplanned, or in countries that lack healthcare resources and education. Some countries have implemented either mandatory or voluntary food fortification of wheat flour and other grains, but many others rely on public health education and one-on-one healthcare practitioner advice. A meta-analysis of global birth prevalence of spina bifida showed that when a national, mandatory program to fortify the diet with folate was compared to countries without such a fortification program, there was a 30% reduction in live births with spina bifida. Some countries reported a greater than 50% reduction. The United States Preventive Services Task Force recommends folic acid as the supplement or fortification ingredient, as forms of folate other than folic acid have not been studied.
A meta-analysis of folate supplementation during pregnancy reported a 28% lower relative risk of newborn congenital heart defects. Prenatal supplementation with folic acid did not appear to reduce the risk of preterm births. One systematic review indicated no effect of folic acid on mortality, growth, body composition, respiratory, or cognitive outcomes of children from birth to 9 years old. There was no relation between maternal folic acid supplementation and an increased risk for childhood asthma.
Fertility
Folate contributes to spermatogenesis. In women, folate is important for oocyte quality and maturation, implantation, placentation, fetal growth and organ development.
Heart disease
One meta-analysis reported that multi-year folic acid supplementation, in amounts in most of the included clinical trials at higher than the upper limit of 1,000 μg/day, reduced the relative risk of cardiovascular disease by a modest 4%. Two older meta-analyses, which would not have incorporated results from newer clinical trials, reported no changes to the risk of cardiovascular disease.
Stroke
The absolute risk of stroke with supplementation decreases from 4.4% to 3.8% (a 10% decrease in relative risk). Two other meta-analyses reported a similar decrease in relative risk. Two of these three were limited to people with pre-existing cardiovascular disease or coronary heart disease. The beneficial result may be associated with lowering circulating homocysteine concentration, as stratified analysis showed that risk was reduced more when there was a larger decrease in homocysteine. The effect was also larger for the studies that were conducted in countries that did not have mandatory grain folic acid fortification. The beneficial effect was larger in the subset of trials that used a lower folic acid supplement compared to higher.
Cancer
Chronically insufficient intake of folate may increase the risk of colorectal, breast, ovarian, pancreatic, brain, lung, cervical, and prostate cancers.
Early after fortification programs were implemented, high intakes were theorized to accelerate the growth of preneoplastic lesions that could lead to cancer, specifically colon cancer. Subsequent meta-analyses of the effects of low versus high dietary folate, elevated serum folate, and supplemental folate in the form of folic acid have reported at times conflicting results. Comparing low to high dietary folate showed a modest but statistically significant reduced risk of colon cancer. For prostate cancer risk, comparing low to high dietary folate showed no effect. A review of trials that involved folic acid dietary supplements reported a statistically significant 24% increase in prostate cancer risk. It was shown that supplementation with folic acid at 1,000 to 2,500 μg/day – the amounts used in many of the cited supplement trials – would result in higher concentrations of serum folate than what is achieved from diets high in food-derived folate. The second supplementation review reported no significant increase or decrease in total cancer incidence, colorectal cancer, other gastrointestinal cancer, genitourinary cancer, lung cancer or hematological malignancies in people who were consuming folic acid supplements. A third supplementation meta-analysis limited to reporting only on colorectal cancer incidence showed that folic acid treatment was not associated with colorectal cancer risk.
Anti-folate chemotherapy
Folate is important for cells and tissues that divide rapidly. Cancer cells divide rapidly, and drugs that interfere with folate metabolism are used to treat cancer. The antifolate drug methotrexate is often used to treat cancer because it inhibits the production of the active tetrahydrofolate (THF) from the inactive dihydrofolate (DHF). However, methotrexate can be toxic, producing side effects such as inflammation in the digestive tract that make eating normally more difficult. Bone marrow depression (inducing leukopenia and thrombocytopenia) and acute kidney and liver failure have been reported.
Folinic acid, under the drug name leucovorin, a form of folate (formyl-THF), can help "rescue" or reverse the toxic effects of methotrexate. Folic acid supplements have little established role in cancer chemotherapy. The supplement of folinic acid in people undergoing methotrexate treatment is to give less rapidly dividing cells enough folate to maintain normal cell functions. The amount of folate given is quickly depleted by rapidly dividing (cancer) cells, so this does not negate the effects of methotrexate.
Neurological disorders
Conversion of homocysteine to methionine requires folate and vitamin B12. Elevated plasma homocysteine and low folate are associated with cognitive impairment, dementia and Alzheimer's disease. Supplementing the diet with folic acid and vitamin B12 lowers plasma homocysteine. However, several reviews reported that supplementation with folic acid alone or in combination with other B vitamins did not prevent development of cognitive impairment nor slow cognitive decline.
Relative risk of autism spectrum disorders (ASDs) was reported reduced by 23% when the maternal diet was supplemented with folic acid during pregnancy. Subset analysis confirmed this among Asian, European and American populations. Cerebral folate deficiency (CFD) has been associated with ASDs. The cerebral folate receptor alpha (FRα) transports 5-methyltetrahydrofolate into the brain. One cause of CFD is autoantibodies that interfere with FRa, and FRa autoantibodies have been reported in ASDs. For individuals with ASD and CFD, meta-analysis reported improvements with treatment with folinic acid, a 5-formyl derivative of tetrahydrofolic acid, for core and associated ASD symptoms.
Some evidence links a shortage of folate with clinical depression. Limited evidence from randomized controlled trials showed using folic acid in addition to selective serotonin reuptake inhibitors (SSRIs) may have benefits. Research found a link between depression and low levels of folate. The exact mechanisms involved in the development of schizophrenia and depression are not entirely clear, but the bioactive folate, methyltetrahydrofolate (5-MTHF), a direct target of methyl donors such as S-adenosyl methionine (SAMe), recycles the inactive dihydrobiopterin (BH2) into tetrahydrobiopterin (BH4), the necessary cofactor in various steps of monoamine synthesis, including that of dopamine and serotonin. BH4 serves a regulatory role in monoamine neurotransmission and is required to mediate the actions of most antidepressants.
Folic acid, B12 and iron
A complex interaction occurs between folic acid, vitamin B12, and iron. A deficiency of folic acid or vitamin B12 may mask the deficiency of iron; so when taken as dietary supplements, the three need to be in balance.
Malaria
Some studies show iron–folic acid supplementation in children under five may result in increased mortality due to malaria; this has prompted the World Health Organization to alter their iron–folic acid supplementation policies for children in malaria-prone areas, such as India.
Absorption, metabolism and excretion
Folate in food is roughly one-third in the form of monoglutamate and two-thirds polyglutamate; the latter is hydrolyzed to monoglutamate via a reaction mediated by folate conjugase at the brush border of enterocytes in the proximal small intestine. Subsequently, intestinal absorption is primarily accomplished by the action of the proton-coupled folate transporter (PCFT) protein coded for by the SLC46A1 gene. This functions best at pH 5.5, which corresponds to the acidic status of the proximal small intestine. PCFT binds to both reduced folates and folic acid. A secondary folate transporter is the reduced folate carrier (RFC), coded for by the SLC19A1 gene. It operates optimally at pH 7.4 in the ileum portion of the small intestine. It has a low affinity for folic acid. Production of the receptor proteins is increased in times of folate deficiency. In addition to a role in intestinal absorption, RFC is expressed in virtually all tissues and is the major route of delivery of folate to cells within the systemic circulation under physiological conditions. When pharmacological amounts of folate are taken as a dietary supplement, absorption also takes place by a passive diffusion-like process. In addition, bacteria in the distal portion of the small intestine and in the large intestine synthesize modest amounts of folate, and there are RFC receptors in the large intestine, so this in situ source may contribute to toward the cellular nutrition and health of the local colonocytes.
The biological activity of folate in the body depends upon dihydrofolate reductase action in the liver which converts folate into tetrahydrofolate (THF). This action is rate-limiting in humans leading to elevated blood concentrations of unmetabolized folic acid when consumption from dietary supplements and fortified foods nears or exceeds the U.S. Tolerable Upper Intake Level of 1,000 μg per day.
The total human body content of folate is estimated to be approximately 15–30 milligrams, with approximately half in the liver. Excretion is via urine and feces. Under normal dietary intake, urinary excretion is mainly as folate cleavage products, but if a dietary supplement is being consumed then there will be intact folate in the urine. The liver produces folate-containing bile, which if not all absorbed in the small intestine, contributes to fecal folate, intact and as cleavage products, which under normal dietary intake has been estimated to be similar in amount to urinary excretion. Fecal content includes what is synthezized by intestinal microflora.
Biosynthesis
Animals, including humans, cannot synthesize (produce) folate and therefore must obtain folate from their diet. All plants and fungi and certain protozoa, bacteria, and archaea can synthesize folate de novo through variations on the same biosynthetic pathway. The folate molecule is synthesized from pterin pyrophosphate, para-aminobenzoic acid (PABA), and glutamate through the action of dihydropteroate synthase and dihydrofolate synthase. Pterin is in turn derived in a series of enzymatically catalyzed steps from guanosine triphosphate (GTP), while PABA is a product of the shikimate pathway.
Bioactivation
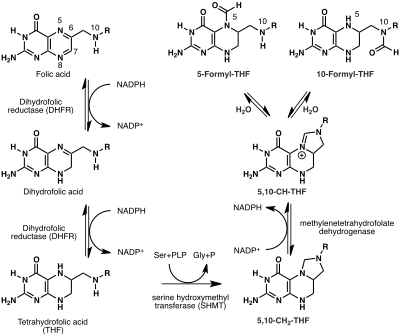
All of the biological functions of folic acid are performed by THF and its methylated derivatives. Hence folic acid must first be reduced to THF. This four electron reduction proceeds in two chemical steps both catalyzed by the same enzyme, dihydrofolate reductase. Folic acid is first reduced to dihydrofolate and then to tetrahydrofolate. Each step consumes one molecule of NADPH (biosynthetically derived from vitamin B3) and produces one molecule of NADP. Mechanistically, hydride is transferred from NADPH to the C6 position of the pteridine ring.
A one-carbon (1C) methyl group is added to tetrahydrofolate through the action of serine hydroxymethyltransferase (SHMT) to yield 5,10-methylenetetrahydrofolate (5,10-CH2-THF). This reaction also consumes serine and pyridoxal phosphate (PLP; vitamin B6) and produces glycine and pyridoxal. A second enzyme, methylenetetrahydrofolate dehydrogenase (MTHFD2) oxidizes 5,10-methylenetetrahydrofolate to an iminium cation which in turn is hydrolyzed to produce 5-formyl-THF and 10-formyl-THF. This series of reactions using the β-carbon atom of serine as the carbon source provide the largest part of the one-carbon units available to the cell.
Alternative carbon sources include formate which by the catalytic action of formate–tetrahydrofolate ligase adds a 1C unit to THF to yield 10-formyl-THF. Glycine, histidine, and sarcosine can also directly contribute to the THF-bound 1C pool.
Drug interference
A number of drugs interfere with the biosynthesis of THF from folic acid. Among them are the antifolate dihydrofolate reductase inhibitors such as the antimicrobial, trimethoprim, the antiprotozoal, pyrimethamine and the chemotherapy drug methotrexate, and the sulfonamides (competitive inhibitors of PABA in the reactions of dihydropteroate synthetase).
Valproic acid, one of the most commonly prescribed epilepsy treatment drugs, also used to treat certain psychological conditions such as bipolar disorder, is a known inhibitor of folic acid, and as such, has been shown to cause birth defects, including neural tube defects, plus increased risk for children having cognitive impairment and autism. There is evidence that folate consumption is protective.
Folate deficiency is common in alcoholics, attributed to both inadequate diet and an inhibition in intestinal processing of the vitamin. Chronic alcohol use inhibits both the digestion process of dietary folate polyglutamates and the uptake phase of liberated folate monoglutamates. The latter is associated with a significant reduction in the level of expression of RFC.
Function
Tetrahydrofolate's main function in metabolism is transporting single-carbon groups (i.e., a methyl group, methylene group, or formyl group). These carbon groups can be transferred to other molecules as part of the modification or biosynthesis of a variety of biological molecules. Folates are essential for the synthesis of DNA, the modification of DNA and RNA, the synthesis of methionine from homocysteine, and various other chemical reactions involved in cellular metabolism. These reactions are collectively known as folate-mediated one-carbon metabolism.
DNA synthesis
Main articles: Purine metabolism and Pyrimidine metabolismFolate derivatives participate in the biosynthesis of both purines and pyrimidines.
Formyl folate is required for two of the steps in the biosynthesis of inosine monophosphate, the precursor to GMP and AMP. Methylenetetrahydrofolate donates the C1 center required for the biosynthesis of dTMP (2′-deoxythymidine-5′-phosphate) from dUMP (2′-deoxyuridine-5′-phosphate). The conversion is catalyzed by thymidylate synthase.
Vitamin B12 activation

Methyl-THF converts vitamin B12 to methyl-B12 (methylcobalamin). Methyl-B12 converts homocysteine, in a reaction catalyzed by homocysteine methyltransferase, to methionine. A defect in homocysteine methyltransferase or a deficiency of B12 may lead to a so-called "methyl-trap" of THF, in which THF converts to methyl-THF, causing a deficiency in folate. Thus, a deficiency in B12 can cause accumulation of methyl-THF, mimicking folate deficiency.
Dietary recommendations
Because of the difference in bioavailability between supplemented folic acid and the different forms of folate found in food, the dietary folate equivalent (DFE) system was established. One DFE is defined as 1 μg of dietary folate. 1 μg of folic acid supplement counts as 1.7 μg DFE. The reason for the difference is that when folic acid is added to food or taken as a dietary supplement with food it is at least 85% absorbed, whereas only about 50% of folate naturally present in food is absorbed.
| Age | Infants | Children and adults | Pregnant women | Lactating women | ||||
|---|---|---|---|---|---|---|---|---|
| (AI) | (UL) | (RDA) | (UL) | (RDA) | (UL) | (RDA) | (UL) | |
| 0–6 months | 65 | None set | – | – | – | – | – | – |
| 7–12 months | 80 | None set | – | – | – | – | – | – |
| 1–3 years | – | – | 150 | 300 | – | – | – | – |
| 4–8 years | – | – | 200 | 400 | – | – | – | – |
| 9–13 years | – | – | 300 | 600 | – | – | – | – |
| 14–18 | – | – | 400 | 800 | 600 | 800 | 500 | 800 |
| 19+ | – | – | 400 | 1000 | 600 | 1000 | 500 | 1000 |
The U.S. Institute of Medicine defines Estimated Average Requirements (EARs), Recommended Dietary Allowances (RDAs), Adequate Intakes (AIs), and Tolerable upper intake levels (ULs) – collectively referred to as Dietary Reference Intakes (DRIs). The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL are defined the same as in the United States. For women and men over age 18, the PRI is set at 330 μg/day. PRI for pregnancy is 600 μg/day, for lactation 500 μg/day. For children ages 1–17 years, the PRIs increase with age from 120 to 270 μg/day. These values differ somewhat from the U.S. RDAs. The United Kingdom's Dietary Reference Value for folate, set by the Committee on Medical Aspects of Food and Nutrition Policy in 1991, is 200 μg/day for adults.
Safety
The risk of toxicity from folic acid is low because folate is a water-soluble vitamin and is regularly removed from the body through urine. One potential issue associated with high doses of folic acid is that it has a masking effect on the diagnosis of pernicious anaemia due to vitamin B12 deficiency, and may even precipitate or exacerbate neuropathy in vitamin B12-deficient individuals. This evidence justified development of a UL for folate. In general, ULs are set for vitamins and minerals when evidence is sufficient. The adult UL of 1,000 μg for folate (and lower for children) refers specifically to folic acid used as a supplement, as no health risks have been associated with high intake of folate from food sources. The EFSA reviewed the safety question and agreed with United States that the UL be set at 1,000 μg. The Japan National Institute of Health and Nutrition set the adult UL at 1,300 or 1,400 μg depending on age.
Reviews of clinical trials that called for long-term consumption of folic acid in amounts exceeding the UL have raised concerns. Excessive amounts derived from supplements are more of a concern than that derived from natural food sources and the relative proportion to vitamin B12 may be a significant factor in adverse effects. One theory is that consumption of large amounts of folic acid leads to detectable amounts of unmetabolized folic acid circulating in blood because the enzyme dihydrofolate reductase that converts folic acid to the biologically active forms is rate limiting. Evidence of a negative health effect of folic acid in blood is not consistent, and folic acid has no known cofactor function that would increase the likelihood of a causal role for free folic acid in disease development. However, low vitamin B12 status in combination with high folic acid intake, in addition to the previously mentioned neuropathy risk, appeared to increase the risk of cognitive impairment in the elderly. Long-term use of folic acid dietary supplements in excess of 1,000 μg/day has been linked to an increase in prostate cancer risk.
Food labeling
For U.S. food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV). For folate labeling purposes, 100% of the Daily Value was 400 μg. As of the 27 May 2016 update, it was kept unchanged at 400 μg. Compliance with the updated labeling regulations was required by 1 January 2020 for manufacturers with US$10 million or more in annual food sales, and by 1 January 2021 for manufacturers with lower volume food sales. A table of the old and new adult daily values is provided at Reference Daily Intake.
European Union regulations require that labels declare energy, protein, fat, saturated fat, carbohydrates, sugars, and salt. Voluntary nutrients may be shown if present in significant amounts. Instead of Daily Values, amounts are shown as percent of Reference Intakes (RIs). For folate, 100% RI was set at 200 μg in 2011.
Deficiency
Main article: Folate deficiencyFolate deficiency can be caused by unhealthy diets that do not include enough vegetables and other folate-rich foods; diseases in which folates are not well absorbed in the digestive system (such as Crohn's disease or celiac disease); some genetic disorders that affect levels of folate; and certain medicines (such as phenytoin, sulfasalazine, or trimethoprim-sulfamethoxazole). Folate deficiency is accelerated by alcohol consumption, possibly by interference with folate transport.
Folate deficiency may lead to glossitis, diarrhea, depression, confusion, anemia, and fetal neural tube and brain defects. Other symptoms include fatigue, gray hair, mouth sores, poor growth, and swollen tongue. Folate deficiency is diagnosed by analyzing a complete blood count (CBC) and plasma vitamin B12 and folate levels. A serum folate of 3 μg/L or lower indicates deficiency. Serum folate level reflects folate status, but erythrocyte folate level better reflects tissue stores after intake. An erythrocyte folate level of 140 μg/L or lower indicates inadequate folate status. Serum folate reacts more rapidly to folate intake than erythrocyte folate.
Since folate deficiency limits cell division, erythropoiesis (production of red blood cells) is hindered. This leads to megaloblastic anemia, which is characterized by large, immature red blood cells. This pathology results from persistently thwarted attempts at normal DNA replication, DNA repair, and cell division, and produces abnormally large red cells called megaloblasts (and hypersegmented neutrophils) with abundant cytoplasm capable of RNA and protein synthesis, but with clumping and fragmentation of nuclear chromatin. Some of these large cells, although immature (reticulocytes), are released early from the marrow in an attempt to compensate for the anemia. Both adults and children need folate to make normal red and white blood cells and prevent anemia, which causes fatigue, weakness, and inability to concentrate.
Increased homocysteine levels suggest tissue folate deficiency, but homocysteine is also affected by vitamin B12 and vitamin B6, renal function, and genetics. One way to differentiate between folate deficiency and vitamin B12 deficiency is by testing for methylmalonic acid (MMA) levels. Normal MMA levels indicate folate deficiency and elevated MMA levels indicate vitamin B12 deficiency. Elevated MMA levels may also be due to the rare metabolic disorder combined malonic and methylmalonic aciduria (CMAMMA).
Folate deficiency is treated with supplemental oral folic acid of 400 to 1000 μg per day. This treatment is very successful in replenishing tissues, even if deficiency was caused by malabsorption. People with megaloblastic anemia need to be tested for vitamin B12 deficiency before treatment with folic acid, because if the person has vitamin B12 deficiency, folic acid supplementation can remove the anemia, but can also worsen neurologic problems. Cobalamin (vitamin B12) deficiency may lead to folate deficiency, which, in turn, increases homocysteine levels and may result in the development of cardiovascular disease or birth defects.
Sources
The United States Department of Agriculture, Agricultural Research Service maintains a food composition database from which folate content in hundreds of foods can be searched as shown in the table. The Food Fortification Initiative lists all countries in the world that conduct fortification programs, and within each country, what nutrients are added to which foods, and whether those programs are voluntary or mandatory. In the US, mandatory fortification of enriched breads, cereals, flours, corn meal, pastas, rice, and other grain products began in January 1998. As of 2023, 140 countries require food fortification with one or more vitamins, with folate required in 69 countries. The most commonly fortified food is wheat flour, followed by maize flour and rice. From country to country, added folic acid amounts range from 0.4 to 5.1 mg/kg, but the great majority are in a more narrow range of 1.0 to 2.5 mg/kg, i.e. 100–250 μg/100g. Folate naturally found in food is susceptible to destruction from high heat cooking, especially in the presence of acidic foods and sauces. It is soluble in water, and so may be lost from foods boiled in water. For foods that are normally consumed cooked, values in the table are for folate naturally occurring in cooked foods.
| Plant sources | Amount as Folate (μg / 100 g) |
|---|---|
| Peanuts | 246 |
| Sunflower seed kernels | 238 |
| Lentils | 181 |
| Chickpeas | 172 |
| Asparagus | 149 |
| Spinach | 146 |
| Lettuce | 136 |
| Peanuts (oil-roasted) | 125 |
| Soybeans | 111 |
| Broccoli | 108 |
| Walnuts | 98 |
| Plant sources | Amount as Folate (μg / 100 g) |
|---|---|
| Peanut butter | 92 |
| Hazelnuts | 88 |
| Avocados | 81 |
| Beets | 80 |
| Kale | 65 |
| Bread (not fortified) | 65 |
| Cabbage | 46 |
| Red bell peppers | 46 |
| Cauliflower | 44 |
| Tofu | 29 |
| Potatoes | 28 |
| Animal sources | Amount as Folate (μg / 100 g) |
|---|---|
| Chicken liver | 578 |
| Calf liver | 331 |
| Cheese | 20–60 |
| Chicken eggs | 44 |
| Salmon | 35 |
| Chicken | 12 |
| Beef | 12 |
| Pork | 8 |
| Yogurt | 8–11 |
| Milk, whole | 5 |
| Butter, salted | 3 |
Food fortification
See also: Food fortificationFolic acid fortification is a process where synthetic folic acid is added to wheat flour or other foods with the intention of promoting public health through increasing blood folate levels in the populace. It is used as it is more stable during processing and storage. After the discovery of the link between insufficient folic acid and neural tube defects, governments and health organizations worldwide made recommendations concerning folic acid supplementation for women intending to become pregnant. Because the neural tube closes in the first four weeks of gestation, often before many women even know they are pregnant, many countries in time decided to implement mandatory food fortification programs. A meta-analysis of global birth prevalence of spina bifida showed that when mandatory fortification was compared to countries with voluntary fortification or no fortification program, there was a 30% reduction in live births with spina bifida, with some countries reporting a greater than 50% reduction.
Folic acid is added to grain products in more than 80 countries, either as required or voluntary fortification, and these fortified products make up a significant source of the population's folate intake. Fortification is controversial, with issues having been raised concerning individual liberty, as well as the theorized health concerns described in the Safety section. In the U.S., there is concern that the federal government mandates fortification but does not provide monitoring of potential undesirable effects of fortification. The Food Fortification Initiative lists all countries in the world that conduct fortification programs, and within each country, what nutrients are added to which foods. The most commonly mandatory fortified vitamin – in 62 countries – is folate; the most commonly fortified food is wheat flour.
Australia and New Zealand
Australia and New Zealand jointly agreed to wheat flour fortification through the Food Standards Australia New Zealand in 2007. The requirement was set at 135 μg of folate per 100 g of bread. Australia implemented the program in 2009. New Zealand was also planning to fortify bread (excluding organic and unleavened varieties) starting in 2009, but then opted to wait until more research was done. The Association of Bakers and the Green Party had opposed mandatory fortification, describing it as "mass medication". Food Safety Minister Kate Wilkinson reviewed the decision to fortify in July 2009, citing as reasons to oppose claims for links between over consumption of folate with increased risk of cancer. In 2012 the delayed mandatory fortification program was revoked and replaced by a voluntary program, with the hope of achieving a 50% bread fortification target.
Canada
Canadian public health efforts focused on promoting awareness of the importance of folic acid supplementation for all women of childbearing age and decreasing socio-economic inequalities by providing practical folic acid support to vulnerable groups of women. Folic acid food fortification became mandatory in 1998, with the fortification of 150 μg of folic acid per 100 grams of enriched flour and uncooked cereal grains. The results of folic acid fortification on the rate of neural tube defects in Canada have been positive, showing a 46% reduction in prevalence of NTDs; the magnitude of reduction was proportional to the prefortification rate of NTDs, essentially removing geographical variations in rates of NTDs seen in Canada before fortification.
United Kingdom
While the Food Standards Agency recommended folic acid fortification, and wheat flour is fortified with iron, folic acid fortification of wheat flour is allowed voluntarily rather than required. A 2018 review by authors based in the United Kingdom strongly recommended that mandatory fortification be reconsidered as a means of reducing the risk of neural tube defects. In November 2024 the UK government announced legislation to require folic acid fortification in bread by the end of 2026.
United States

In 1996, the United States Food and Drug Administration (FDA) published regulations requiring the addition of folic acid to enriched breads, cereals, flours, corn meals, pastas, rice, and other grain products. This ruling took effect on 1 January 1998, and was specifically targeted to reduce the risk of neural tube birth defects in newborns. There were concerns expressed that the amount of folate added was insufficient.
The fortification program was expected to raise a person's folic acid intake level by 70–130 μg/day; however, an increase of almost double that amount was actually observed. This could be from the fact that many foods are fortified by 160–175% over the required amount. Much of the elder population take supplements that add 400 μg to their daily folic acid intake. This is a concern because 70–80% of the population have detectable levels of unmetabolized folic acid in their blood, a consequence of folic acid supplementation and fortification. However, at blood concentrations achieved via food fortification, folic acid has no known cofactor function that would increase the likelihood of a causal role for free folic acid in disease development.
The U.S. National Center for Health Statistics conducts the biannual National Health and Nutrition Examination Survey (NHANES) to assess the health and nutritional status of adults and children in the United States. Some results are reported as What We Eat In America. The 2013–2014 survey reported that for adults ages 20 years and older, men consumed an average of 249 μg/day folate from food plus 207 μg/day of folic acid from consumption of fortified foods, for a combined total of 601 μg/day of dietary folate equivalents (DFEs because each microgram of folic acid counts as 1.7 μg of food folate). For women, the values are 199, 153 and 459 μg/day, respectively. This means that fortification led to a bigger increase in folic acid intake than first projected, and that more than half the adults are consuming more than the RDA of 400 μg (as DFEs). Even so, fewer than half of pregnant women are exceeding the pregnancy RDA of 600 μg/day.
Before folic acid fortification, about 4,100 pregnancies were affected by a neural tube defect each year in the United States. The Centers for Disease Control and Prevention reported in 2015 that since the addition of folic acid in grain-based foods as mandated by the FDA, the rate of neural tube defects dropped by 35%. This translates to an annual saving in total direct costs of approximately $508 million for the NTD-affected births that were prevented.
History
Further information: Vitamin § HistoryIn the 1920s, scientists believed folate deficiency and anemia were the same condition. In 1931, researcher Lucy Wills made a key observation that led to the identification of folate as the nutrient required to prevent anemia during pregnancy. Wills demonstrated that anemia could be reversed with brewer's yeast. In the late 1930s, folate was identified as the corrective substance in brewer's yeast. It was first isolated via extraction from spinach leaves by Herschel K. Mitchell, Esmond E. Snell, and Roger J. Williams in 1941. The term "folic" is from the Latin word folium (which means leaf) because it was found in dark-green leafy vegetables. Historic names included L. casei factor, vitamin Bc after research done in chicks and vitamin M after research done in monkeys.
Bob Stokstad isolated the pure crystalline form in 1943, and was able to determine its chemical structure while working at the Lederle Laboratories of the American Cyanamid Company. This historical research project, of obtaining folic acid in a pure crystalline form in 1945, was done by the team called the "folic acid boys", under the supervision and guidance of Director of Research Dr. Yellapragada Subbarow, at the Lederle Lab, Pearl River, New York. This research subsequently led to the synthesis of the antifolate aminopterin, which was used to treat childhood leukemia by Sidney Farber in 1948.
In the 1950s and 1960s, scientists began to discover the biochemical mechanisms of action for folate. In 1960, researchers linked folate deficiency to risk of neural tube defects. In the late 1990s, the U.S. and Canadian governments decided that despite public education programs and the availability of folic acid supplements, there was still a challenge for women of child-bearing age to meet the daily folate recommendations, which is when those two countries implemented folate fortification programs. As of December 2018, 62 countries mandated food fortification with folic acid.
Animals
Veterinarians may test cats and dogs if a risk of folate deficiency is indicated. Cats with exocrine pancreatic insufficiency, more so than dogs, may have low serum folate. In dog breeds at risk for cleft lip and cleft palate dietary folic acid supplementation significantly decreased incidence.
References
- ^ "Folate – Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 29 March 2021. Archived from the original on 2 April 2011. Retrieved 29 April 2022.
- ^ Welch AD (1983). "Folic acid: discovery and the exciting first decade". Perspect. Biol. Med. 27 (1): 64–75. doi:10.1353/pbm.1983.0006. PMID 6359053. S2CID 31993927.
- ^ "Folic Acid". Drugs.com. American Society of Health-System Pharmacists. 1 January 2010. Archived from the original on 8 August 2017. Retrieved 1 September 2016.
- "Folic Acid". The PubChem Project. Archived from the original on 7 April 2014.
- ^ "Folic Acid". ChemSrc. Archived from the original on 28 August 2021. Retrieved 12 April 2018.
- ^ "Folate". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2014. Archived from the original on 19 August 2021. Retrieved 17 March 2018.
Folate is a water-soluble B-vitamin, which is also known as vitamin B9 or folacin.
- ^ Choi JH, Yates Z, Veysey M, Heo YR, Lucock M (December 2014). "Contemporary issues surrounding folic Acid fortification initiatives". Prev Nutr Food Sci. 19 (4): 247–60. doi:10.3746/pnf.2014.19.4.247. PMC 4287316. PMID 25580388.
- ^ West AA, Caudill MA, Bailey LB (2020). "Folate". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 273–88. ISBN 978-0-323-66162-1.
- Pommerville JC (2009). Alcamo's Fundamentals of Microbiology: Body Systems. Jones & Bartlett Publishers. p. 511. ISBN 978-0-7637-8712-7. Archived from the original on 8 September 2017.
- Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, et al. (January 2017). "Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement". JAMA. 317 (2): 183–189. doi:10.1001/jama.2016.19438. PMID 28097362. S2CID 205077749.
- ^ Wald NJ, Morris JK, Blakemore C (2018). "Public health failure in the prevention of neural tube defects: time to abandon the tolerable upper intake level of folate". Public Health Reviews. 39: 2. doi:10.1186/s40985-018-0079-6. PMC 5809909. PMID 29450103.
- ^ Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB (August 2016). "Folic Acid Supplementation and the Risk of Cardiovascular Diseases: A Meta-Analysis of Randomized Controlled Trials" (PDF). Journal of the American Heart Association. 5 (8): e003768. doi:10.1161/JAHA.116.003768. PMC 5015297. PMID 27528407. Archived (PDF) from the original on 27 April 2021. Retrieved 4 November 2018.
- ^ Wien TN, Pike E, Wisløff T, Staff A, Smeland S, Klemp M (January 2012). "Cancer risk with folic acid supplements: a systematic review and meta-analysis". BMJ Open. 2 (1): e000653. doi:10.1136/bmjopen-2011-000653. PMC 3278486. PMID 22240654.
- Marino BS, Fine KS (2009). Blueprints Pediatrics. Lippincott Williams & Wilkins. p. 131. ISBN 978-0-7817-8251-7. Archived from the original on 8 September 2017.
- Fardous AM, Heydari AR (November 2023). "Uncovering the Hidden Dangers and Molecular Mechanisms of Excess Folate: A Narrative Review". Nutrients. 15 (21): 4699. doi:10.3390/nu15214699. PMC 10648405. PMID 37960352.
- ^ Pond WG, Nichols BL, Brown DL (2009). Adequate Food for All: Culture, Science, and Technology of Food in the 21st Century. CRC Press. p. 148. ISBN 978-1-4200-7754-4.
Folic acid's discovery started in 1931...
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- "Folic Acid Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Archived from the original on 8 July 2020. Retrieved 30 August 2024.
- ^ Chambers Concise Dictionary. Allied Publishers. 2004. p. 451. ISBN 978-81-86062-36-4. Archived from the original on 8 September 2017.
- "Folic Acid". NIH LiverTox. 2 June 2017. Archived from the original on 7 January 2017.
- ^ "FAQ's Folic Acid". CDC. 16 December 2016. Archived from the original on 10 July 2017. Retrieved 7 July 2017.
- ^ Moss GP (1986). "Nomenclature and symbols for folic acid and related compounds". IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Archived from the original on 30 November 2020. Retrieved 15 December 2019.
Folate and folic acid are the preferred synonyms for pteroylglutamate and pteroylglutamic acid, respectively.
- "Folic Acid". MedlinePlus. U.S. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services. Archived from the original on 31 July 2020. Retrieved 15 December 2019.
- "Folic acid". Chemical Entities of Biological Interest (ChEBI). European Bioinformatics Institute. Archived from the original on 31 July 2020. Retrieved 15 December 2019.
- Combs JR GF, McClung JP (2016). "Chapter 17: Folate". The Vitamins: Fundamental Aspects in Nutrition and Health (Fifth ed.). Academic Press. pp. 400–401. ISBN 978-0-12-802983-1. Archived from the original on 12 January 2023. Retrieved 15 December 2019.
The term folate is the generic descriptor for folic acid (pteroylmonoglutamic acid or pteroylglutamic acid) and related compounds exhibiting the biological activity of folic acid.
- "Folic acid in diet". MedlinePlus. U.S. National Library of Medicine, National Institutes of Health, U.S. Department of Health and Human Services. Archived from the original on 31 July 2020. Retrieved 15 December 2019.
- Zheng Y, Cantley LC (February 2019). "Toward a better understanding of folate metabolism in health and disease". The Journal of Experimental Medicine. 216 (2): 253–266. doi:10.1084/jem.20181965. PMC 6363433. PMID 30587505.
- Berry RJ, Bailey L, Mulinare J, Bower C (2010). "Fortification of flour with folic acid". Food Nutr Bull. 31 (1 Suppl): S22–S35. doi:10.1177/15648265100311S103. PMID 20629350. S2CID 36706350.
- Wilson RD, Wilson RD, Audibert F, Brock JA, Carroll J, Cartier L, et al. (June 2015). "Pre-conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid-Sensitive Congenital Anomalies". Journal of Obstetrics and Gynaecology Canada. 37 (6): 534–52. doi:10.1016/s1701-2163(15)30230-9. PMID 26334606.
- "Folic Acid". CDC. 19 April 2021. Archived from the original on 15 July 2023. Retrieved 20 December 2021.
- ^ "Map: Count of Nutrients In Fortification Standards". Global Fortification Data Exchange. Archived from the original on 11 April 2019. Retrieved 30 April 2019.
- ^ Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, et al. (January 2016). "Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis". American Journal of Public Health. 106 (1): e24-34. doi:10.2105/AJPH.2015.302902. PMC 4695937. PMID 26562127.
- ^ Castillo-Lancellotti C, Tur JA, Uauy R (2013). "Impact of folic acid fortification of flour on neural tube defects: a systematic review". Public Health Nutr. 16 (5): 901–911. doi:10.1017/S1368980012003576. PMC 10271422. PMID 22850218.
- Feng Y, Wang S, Chen R, Tong X, Wu Z, Mo X (February 2015). "Maternal folic acid supplementation and the risk of congenital heart defects in offspring: a meta-analysis of epidemiological observational studies". Scientific Reports. 5: 8506. Bibcode:2015NatSR...5.8506F. doi:10.1038/srep08506. PMC 4330542. PMID 25687545.
- Fekete K, Berti C, Trovato M, Lohner S, Dullemeijer C, Souverein OW, et al. (September 2012). "Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation". Nutrition Journal. 11: 75. doi:10.1186/1475-2891-11-75. PMC 3499376. PMID 22992251.
- Saccone G, Berghella V (April 2016). "Folic acid supplementation in pregnancy to prevent preterm birth: a systematic review and meta-analysis of randomized controlled trials". European Journal of Obstetrics, Gynecology, and Reproductive Biology. 199: 76–81. doi:10.1016/j.ejogrb.2016.01.042. PMID 26901401.
- Devakumar D, Fall CH, Sachdev HS, Margetts BM, Osmond C, Wells JC, et al. (June 2016). "Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis". BMC Medicine. 14: 90. doi:10.1186/s12916-016-0633-3. PMC 4910255. PMID 27306908.
- Crider KS, Cordero AM, Qi YP, Mulinare J, Dowling NF, Berry RJ (November 2013). "Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis". The American Journal of Clinical Nutrition. 98 (5): 1272–81. doi:10.3945/ajcn.113.065623. PMC 5369603. PMID 24004895.
- ^ Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP (March–April 2007). "The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility". Human Reproduction Update. 13 (2): 163–74. doi:10.1093/humupd/dml054. PMID 17099205.
- Yang HT, Lee M, Hong KS, Ovbiagele B, Saver JL (December 2012). "Efficacy of folic acid supplementation in cardiovascular disease prevention: an updated meta-analysis of randomized controlled trials". Eur. J. Intern. Med. 23 (8): 745–54. doi:10.1016/j.ejim.2012.07.004. PMID 22884409.
- Bazzano LA (August 2011). "No effect of folic acid supplementation on cardiovascular events, cancer or mortality after 5 years in people at increased cardiovascular risk, although homocysteine levels are reduced". Evidence-Based Medicine. 16 (4): 117–8. doi:10.1136/ebm1204. PMID 21402567. S2CID 20470125.
- ^ Tian T, Yang KQ, Cui JG, Zhou LL, Zhou XL (October 2017). "Folic Acid Supplementation for Stroke Prevention in Patients With Cardiovascular Disease". Am. J. Med. Sci. 354 (4): 379–387. doi:10.1016/j.amjms.2017.05.020. PMID 29078842. S2CID 3500861.
- ^ Zhao M, Wu G, Li Y, Wang X, Hou FF, Xu X, et al. (May 2017). "Meta-analysis of folic acid efficacy trials in stroke prevention: Insight into effect modifiers". Neurology. 88 (19): 1830–1838. doi:10.1212/WNL.0000000000003909. PMID 28404799. S2CID 325155.
- Jägerstad M (October 2012). "Folic acid fortification prevents neural tube defects and may also reduce cancer risks". Acta Paediatrica. 101 (10): 1007–12. doi:10.1111/j.1651-2227.2012.02781.x. PMID 22783992. S2CID 3458384.
- Weinstein SJ, Hartman TJ, Stolzenberg-Solomon R, Pietinen P, Barrett MJ, Taylor PR, et al. (November 2003). "Null association between prostate cancer and serum folate, vitamin B(6), vitamin B(12), and homocysteine". Cancer Epidemiology, Biomarkers & Prevention. 12 (11 Pt 1): 1271–2. PMID 14652294. Archived from the original on 22 February 2017.
- ^ Chustecka Z (2009). "Folic-acid fortification of flour and increased rates of colon cancer". Medscape. Archived from the original on 25 November 2010. Retrieved 9 November 2009.
- ^ Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, et al. (July 2007). "A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis". Cancer Epidemiology, Biomarkers & Prevention. 16 (7): 1325–9. doi:10.1158/1055-9965.EPI-07-0329. PMID 17626997.
- Kim DH, Smith-Warner SA, Spiegelman D, Yaun SS, Colditz GA, Freudenheim JL, et al. (November 2010). "Pooled analyses of 13 prospective cohort studies on folate intake and colon cancer". Cancer Causes Control. 21 (11): 1919–30. doi:10.1007/s10552-010-9620-8. PMC 3082430. PMID 20820900.
- Wang R, Zheng Y, Huang JY, Zhang AQ, Zhou YH, Wang JN (December 2014). "Folate intake, serum folate levels, and prostate cancer risk: a meta-analysis of prospective studies". BMC Public Health. 14 (1): 1326. doi:10.1186/1471-2458-14-1326. PMC 4320532. PMID 25543518.
- Tio M, Andrici J, Cox MR, Eslick GD (September 2014). "Folate intake and the risk of prostate cancer: a systematic review and meta-analysis". Prostate Cancer Prostatic Dis. 17 (3): 213–9. doi:10.1038/pcan.2014.16. PMID 24819234. S2CID 27184844.
- ^ Qin X, Cui Y, Shen L, Sun N, Zhang Y, Li J, et al. (September 2013). "Folic acid supplementation and cancer risk: a meta-analysis of randomized controlled trials". Int. J. Cancer. 133 (5): 1033–41. doi:10.1002/ijc.28038. PMID 23338728. S2CID 19830376.
- Qin T, Du M, Du H, Shu Y, Wang M, Zhu L (July 2015). "Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials". Sci Rep. 5: 12044. Bibcode:2015NatSR...512044Q. doi:10.1038/srep12044. PMC 4487230. PMID 26131763.
- Kamen B (October 1997). "Folate and antifolate pharmacology". Seminars in Oncology. 24 (5 Suppl 18): S18-30-S18-39. PMID 9420019.
- Gonen N, Assaraf YG (August 2012). "Antifolates in cancer therapy: structure, activity and mechanisms of drug resistance". Drug Resistance Updates: Reviews and Commentaries in Antimicrobial and Anticancer Chemotherapy. 15 (4): 183–210. doi:10.1016/j.drup.2012.07.002. PMID 22921318.
- Rubio IT, Cao Y, Hutchins LF, Westbrook KC, Klimberg VS (May 1998). "Effect of glutamine on methotrexate efficacy and toxicity". Annals of Surgery. 227 (5): 772–8, discussion 778–80. doi:10.1097/00000658-199805000-00018. PMC 1191365. PMID 9605669.
- Wolff JE, Hauch H, Kühl J, Egeler RM, Jürgens H (1998). "Dexamethasone increases hepatotoxicity of MTX in children with brain tumors". Anticancer Research. 18 (4B): 2895–9. PMID 9713483.
- Kepka L, De Lassence A, Ribrag V, Gachot B, Blot F, Theodore C, et al. (March 1998). "Successful rescue in a patient with high dose methotrexate-induced nephrotoxicity and acute renal failure". Leukemia & Lymphoma. 29 (1–2): 205–9. doi:10.3109/10428199809058397. PMID 9638991.
- Branda RF, Nigels E, Lafayette AR, Hacker M (October 1998). "Nutritional folate status influences the efficacy and toxicity of chemotherapy in rats". Blood. 92 (7): 2471–6. doi:10.1182/blood.V92.7.2471. PMID 9746787.
- Shiroky JB (November 1997). "The use of folates concomitantly with low-dose pulse methotrexate". Rheumatic Disease Clinics of North America. 23 (4): 969–80. doi:10.1016/S0889-857X(05)70369-0. PMID 9361164.
- Keshava C, Keshava N, Whong WZ, Nath J, Ong TM (February 1998). "Inhibition of methotrexate-induced chromosomal damage by folinic acid in V79 cells". Mutation Research. 397 (2): 221–8. Bibcode:1998MRFMM.397..221K. doi:10.1016/S0027-5107(97)00216-9. PMID 9541646.
- Shen L, Ji HF (2015). "Associations between Homocysteine, Folic Acid, Vitamin B12 and Alzheimer's Disease: Insights from Meta-Analyses". J. Alzheimers Dis. 46 (3): 777–90. doi:10.3233/JAD-150140. PMID 25854931.
- ^ Ford AH, Almeida OP (2012). "Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials". J. Alzheimers Dis. 29 (1): 133–49. doi:10.3233/JAD-2012-111739. PMID 22232016.
- Li MM, Yu JT, Wang HF, Jiang T, Wang J, Meng XF, et al. (2014). "Efficacy of vitamins B supplementation on mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis". Curr Alzheimer Res. 11 (9): 844–52. PMID 25274113.
- Wald DS, Kasturiratne A, Simmonds M (June 2010). "Effect of folic acid, with or without other B vitamins, on cognitive decline: meta-analysis of randomized trials". Am. J. Med. 123 (6): 522–527.e2. doi:10.1016/j.amjmed.2010.01.017. PMID 20569758.
- Wang M, Li K, Zhao D, Li L (2017). "The association between maternal use of folic acid supplements during pregnancy and risk of autism spectrum disorders in children: a meta-analysis". Mol Autism. 8: 51. doi:10.1186/s13229-017-0170-8. PMC 5625821. PMID 29026508.
- Rossignol DA, Frye RE (November 2021). "Cerebral folate deficiency, folate receptor alpha autoantibodies and leucovorin (folinic acid) treatment in autism spectrum disorders: A systematic review and meta-analysis". J Pers Med. 11 (11): 1141. doi:10.3390/jpm11111141. PMC 8622150. PMID 34834493.
- Coppen A, Bolander-Gouaille C (January 2005). "Treatment of depression: time to consider folic acid and vitamin B12". Journal of Psychopharmacology. 19 (1): 59–65. doi:10.1177/0269881105048899. PMID 15671130. S2CID 4828454.
- Taylor MJ, Carney SM, Goodwin GM, Geddes JR (June 2004). "Folate for depressive disorders: systematic review and meta-analysis of randomized controlled trials". Journal of Psychopharmacology. 18 (2): 251–6. doi:10.1177/0269881104042630. PMID 15260915. S2CID 9107724.
- Gilbody S, Lewis S, Lightfoot T (January 2007). "Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review". American Journal of Epidemiology. 165 (1): 1–13. doi:10.1093/aje/kwj347. PMID 17074966.
- Gilbody S, Lightfoot T, Sheldon T (July 2007). "Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity". Journal of Epidemiology and Community Health. 61 (7): 631–7. doi:10.1136/jech.2006.050385. PMC 2465760. PMID 17568057.
- Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H (December 2009). "One-carbon metabolism and schizophrenia: current challenges and future directions". Trends in Molecular Medicine. 15 (12): 562–70. doi:10.1016/j.molmed.2009.10.001. PMID 19896901.
- Vreugdenhil G, Wognum AW, van Eijk HG, Swaak AJ (February 1990). "Anaemia in rheumatoid arthritis: the role of iron, vitamin B12, and folic acid deficiency, and erythropoietin responsiveness". Annals of the Rheumatic Diseases. 49 (2): 93–8. doi:10.1136/ard.49.2.93. PMC 1003985. PMID 2317122.
- Allen RH, Stabler SP, Savage DG, Lindenbaum J (June 1990). "Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations". American Journal of Hematology. 34 (2): 90–8. doi:10.1002/ajh.2830340204. PMID 2339683. S2CID 23092095.
- Reynolds E (November 2006). "Vitamin B12, folic acid, and the nervous system". The Lancet. Neurology. 5 (11): 949–60. doi:10.1016/S1474-4422(06)70598-1. PMID 17052662. S2CID 2165819.
- Pasricha S, Shet A, Sachdev HP, Shet AS (October 2009). "Risks of routine iron and folic acid supplementation for young children" (PDF). Indian Pediatrics. 46 (10): 857–66. PMID 19887691. Archived (PDF) from the original on 12 June 2010.
- Alpers DH (July 2016). "Absorption and blood/cellular transport of folate and cobalamin: Pharmacokinetic and physiological considerations". Biochimie. 126: 52–6. doi:10.1016/j.biochi.2015.11.006. PMC 4867132. PMID 26586110.
- ^ Said HM (August 2011). "Intestinal absorption of water-soluble vitamins in health and disease". Biochem J. 437 (3): 357–72. doi:10.1042/BJ20110326. PMC 4049159. PMID 21749321.
- ^ Visentin M, Diop-Bove N, Zhao R, Goldman ID (2014). "The intestinal absorption of folates". Annu Rev Physiol. 76: 251–74. doi:10.1146/annurev-physiol-020911-153251. PMC 3982215. PMID 24512081.
- Bailey SW, Ayling JE (September 2009). "The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake". Proceedings of the National Academy of Sciences of the United States of America. 106 (36): 15424–9. Bibcode:2009PNAS..10615424B. doi:10.1073/pnas.0902072106. PMC 2730961. PMID 19706381.
- ^ Rossi M, Amaretti A, Raimondi S (January 2011). "Folate production by probiotic bacteria". Nutrients. 3 (1): 118–34. doi:10.3390/nu3010118. PMC 3257725. PMID 22254078.
- ^ Carmen AJ, Carlos M (2008). "Chapter 2 – Antimetabolites". Medicinal Chemistry of Anticancer Drugs. pp. 9–52. doi:10.1016/B978-0-444-52824-7.00002-0. ISBN 978-0-444-52824-7.
Figure 2.27: Biotransformation of folic acid into folinic acids
- "EC 1.5.1.3". Us.expasy.org. Archived from the original on 13 June 2011. Retrieved 9 September 2012.
- Benkovic SJ, Hammes-Schiffer S (August 2003). "A perspective on enzyme catalysis". Science. 301 (5637): 1196–202. Bibcode:2003Sci...301.1196B. doi:10.1126/science.1085515. PMID 12947189. S2CID 7899320.
- Christensen KE, Mackenzie RE (2008). "Mitochondrial methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase, and formyltetrahydrofolate synthetases". Vitamins and Hormones. 79: 393–410. doi:10.1016/S0083-6729(08)00414-7. PMID 18804703.
- Stover P, Schirch V (August 1990). "Serine hydroxymethyltransferase catalyzes the hydrolysis of 5,10-methenyltetrahydrofolate to 5-formyltetrahydrofolate". The Journal of Biological Chemistry. 265 (24): 14227–33. doi:10.1016/S0021-9258(18)77290-6. PMID 2201683.
- Ducker GS, Rabinowitz JD (January 2017). "One-Carbon Metabolism in Health and Disease". Cell Metabolism. 25 (1): 27–42. doi:10.1016/j.cmet.2016.08.009. PMC 5353360. PMID 27641100.
- Rajagopalan PT, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG (October 2002). "Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13481–6. Bibcode:2002PNAS...9913481R. doi:10.1073/pnas.172501499. PMC 129699. PMID 12359872.
- McGuire JJ (2003). "Anticancer antifolates: current status and future directions". Current Pharmaceutical Design. 9 (31): 2593–613. doi:10.2174/1381612033453712. PMID 14529544.
- Boothe DM. "Sulfonamides and Sulfonamide Combinations". Merck Veterinary Manual. Kenilworth, NJ: Merck & Co., Inc. Archived from the original on 7 August 2020. Retrieved 10 October 2019.
- Verrotti A, Tana M, Pelliccia P, Chiarelli F, Latini G (March 2006). "Recent advances on neural tube defects with special reference to Valproic Acid". Endocr Metab Immune Disord Drug Targets. 6 (1): 25–31. doi:10.2174/187153006776056657. PMID 16611162.
- Tanoshima M, Kobayashi T, Tanoshima R, Beyene J, Koren G, Ito S (October 2015). "Risks of congenital malformations in offspring exposed to valproic acid in utero: A systematic review and cumulative meta-analysis". Clin. Pharmacol. Ther. 98 (4): 417–41. doi:10.1002/cpt.158. PMID 26044279. S2CID 205715968.
- Veroniki AA, Rios P, Cogo E, Straus SE, Finkelstein Y, Kealey R, et al. (July 2017). "Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis". BMJ Open. 7 (7): e017248. doi:10.1136/bmjopen-2017-017248. PMC 5642793. PMID 28729328.
- Naderi N, House JD (2018). "Chapter Five – Recent Developments in Folate Nutrition". Advances in Food and Nutrition Research. 83. Elsevier: 195–213. doi:10.1016/bs.afnr.2017.12.006. PMID 29477222.
- Lan X, Field MS, Stover PJ (November 2018). "Cell cycle regulation of folate-mediated one-carbon metabolism". Wiley Interdiscip Rev Syst Biol Med. 10 (6): e1426. doi:10.1002/wsbm.1426. PMID 29889360. S2CID 47014043.
- Froese DS, Fowler B, Baumgartner MR (July 2019). "Vitamin B12, folate, and the methionine remethylation cycle-biochemistry, pathways, and regulation". Journal of Inherited Metabolic Disease. 42 (4): 673–685. doi:10.1002/jimd.12009. PMID 30693532.
- ^ Hoffbrand AV, Weir DG (June 2001). "The history of folic acid". British Journal of Haematology. 113 (3): 579–89. doi:10.1046/j.1365-2141.2001.02822.x. PMID 11380441. S2CID 22925228.
- ^ Institute of Medicine (1998). "Folate". Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: The National Academies Press. pp. 196–305. ISBN 978-0-309-06554-2. Archived from the original on 25 September 2019. Retrieved 25 September 2019.
- "Overview on Dietary Reference Values for the EU population as derived by the EFSA Panel on Dietetic Products, Nutrition and Allergies" (PDF). 2017. Archived (PDF) from the original on 28 August 2017.
- "Nutrition Requirements" (PDF). British Nutrition Foundation. Archived from the original (PDF) on 11 February 2019. Retrieved 8 July 2018.
- "Tolerable Upper Intake Levels For Vitamins And Minerals" (PDF). European Food Safety Authority. 2006. Archived (PDF) from the original on 19 September 2017. Retrieved 16 May 2016.
- Shibata K, Fukuwatari T, Imai E, Hayakawa T, Watanabe F, Takimoto H, et al. (2013). "Dietary Reference Intakes for Japanese 2010: Water-Soluble Vitamins". Journal of Nutritional Science and Vitaminology. 2013 (59): S67–S82. doi:10.3177/jnsv.59.S67. Archived from the original on 14 September 2019. Retrieved 27 September 2018.
- "Folic Acid Overload?". Tufts Health & Nutrition Letter. 10 September 2019. Retrieved 18 October 2021.
- ^ Obeid R, Herrmann W (October 2012). "The emerging role of unmetabolized folic acid in human diseases: myth or reality?". Current Drug Metabolism. 13 (8): 1184–95. doi:10.2174/138920012802850137. PMID 22746304.
- ^ Smith AD (January 2007). "Folic acid fortification: the good, the bad, and the puzzle of vitamin B-12". The American Journal of Clinical Nutrition. 85 (1): 3–5. doi:10.1093/ajcn/85.1.3. PMID 17209170.
- "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 7 October 2021. Retrieved 30 August 2017.
- "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- "Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 27 May 2016. Archived from the original on 6 May 2018. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- "Industry Resources on the Changes to the Nutrition Facts Label". U.S. Food and Drug Administration (FDA). 21 December 2018. Archived from the original on 25 December 2020. Retrieved 16 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- "Regulation (EU) No 1169/2011 of the European Parliament and of the Council". Official Journal of the European Union. 22 (11): 18–63. 2011. Archived from the original on 26 July 2017. Retrieved 26 September 2018.
- ^ "Folate deficiency: MedlinePlus Medical Encyclopedia". nlm.nih.gov. Archived from the original on 17 November 2015. Retrieved 16 November 2015.
- Hamid A, Wani NA, Kaur J (April 2009). "New perspectives on folate transport in relation to alcoholism-induced folate malabsorption–association with epigenome stability and cancer development". The FEBS Journal. 276 (8): 2175–91. doi:10.1111/j.1742-4658.2009.06959.x. PMID 19292860. S2CID 8591709.
- Lohner S, Fekete K, Berti C, Hermoso M, Cetin I, Koletzko B, et al. (December 2012). "Effect of folate supplementation on folate status and health outcomes in infants, children and adolescents: a systematic review". International Journal of Food Sciences and Nutrition. 63 (8): 1014–20. doi:10.3109/09637486.2012.683779. PMID 22574624. S2CID 26868696.
- Lieberman M, Marks AD, Smith C (2007). Marks' Essential Medical Biochemistry, First edition. Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-9340-7.
- Zittoun J (June 1993). "Anémies par trouble du métabolisme des folates, de la vitamine B12 et des transcobalamines" [Anemias due to disorder of folate, vitamin B12 and transcobalamin metabolism]. La Revue du Praticien (in French). 43 (11): 1358–63. PMID 8235383.
- "Folate and Your Health – HealthLinkBC File #68g". Healthlink British Columbia. Archived from the original on 9 July 2012. Retrieved 9 September 2012.
- Sloan JL, Johnston JJ, Manoli I, Chandler RJ, Krause C, Carrillo-Carrasco N, et al. (August 2011). "Exome sequencing identifies ACSF3 as a cause of combined malonic and methylmalonic aciduria". Nat Genet. 43 (9): 883–86. doi:10.1038/ng.908. PMC 3163731. PMID 21841779.
- de Sain-van der Velden MG, van der Ham M, Jans JJ, Visser G, Prinsen HC, Verhoeven-Duif NM, et al. (February 2016). "A New Approach for Fast Metabolic Diagnostics in CMAMMA". JIMD Rep. JIMD Reports. 30: 15–22. doi:10.1007/8904_2016_531. ISBN 978-3-662-53680-3. PMC 5110436. PMID 26915364.
- Varela-Moreiras G, Murphy MM, Scott JM (May 2009). "Cobalamin, folic acid, and homocysteine". Nutrition Reviews. 67 (Suppl 1): S69-72. doi:10.1111/j.1753-4887.2009.00163.x. hdl:2262/34510. PMID 19453682.
- ^ "Folate content in micrograms per 100 g, All Foods; USDA Food Composition Databases". United States Department of Agriculture, Agricultural Research Service. Release 28. 7 May 2019. Retrieved 27 May 2019.
- ^ "Why fortify?". Food Fortification Initiative. 2017. Archived from the original on 4 April 2017. Retrieved 30 April 2019.
- "Effects of Cooking on Vitamins (Table)". Beyondveg.com. Archived from the original on 16 October 2012. Retrieved 30 April 2019.
- Dietrich M, Brown CJ, Block G (August 2005). "The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States". Journal of the American College of Nutrition. 24 (4): 266–74. doi:10.1080/07315724.2005.10719474. PMID 16093404. S2CID 24699315.
- "Folic Acid Fortification". Food Standards Australia New Zealand. 2016. Archived from the original on 26 September 2018. Retrieved 25 September 2018.
- "Work Starts on Wilkinson's Mass Medication Plan" (Press release). Association Of Bakers. 8 July 2009. Archived from the original on 10 July 2009. Retrieved 13 July 2009.
- "NZ should push pause on folic fortification" (Press release). Green Party. 9 July 2009. Archived from the original on 10 July 2009. Retrieved 13 July 2009.
- NZPA (8 July 2009). "Bakers, Govt battle over folic acid". NZ Herald. Archived from the original on 14 February 2012. Retrieved 13 July 2009.
- Houghton LA (August 2014). "A country left behind: folic acid food fortification policy in New Zealand". The New Zealand Medical Journal. 127 (1399): 6–9. PMID 25145300.
- "Welcome to the Health Canada Web site". Hc-sc.gc.ca. Archived from the original on 10 September 2012. Retrieved 9 September 2012.
- De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, et al. (July 2007). "Reduction in neural-tube defects after folic acid fortification in Canada". The New England Journal of Medicine. 357 (2): 135–42. doi:10.1056/NEJMoa067103. PMID 17625125.
- FSA (17 May 2007). "Board recommends mandatory fortification". Archived from the original on 24 June 2007. Retrieved 18 May 2007.
- "Backing for folic acid in bread". BBC News. 17 May 2007. Archived from the original on 18 June 2007. Retrieved 18 May 2007.
- BBC Experts back folic acid in flour Archived 18 August 2007 at the Wayback Machine 11 May 2007
- "Why fortify?". Food Fortification Initiative. 2017. Archived from the original on 4 April 2017. Retrieved 4 April 2017.
- "Birth defects prevented by fortifying flour with folic acid".
- "Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Final Rule. 21 CFR Parts 136, 137, and 139" (PDF). Federal Register. March 1996. pp. 8781–89. Archived (PDF) from the original on 6 October 2019. Retrieved 6 October 2019.
- ^ Crandall BF, Corson VL, Evans MI, Goldberg JD, Knight G, Salafsky IS (July 1998). "American College of Medical Genetics statement on folic acid: fortification and supplementation". American Journal of Medical Genetics. 78 (4): 381. doi:10.1002/(SICI)1096-8628(19980724)78:4<381::AID-AJMG16>3.0.CO;2-E. PMID 9714444.
- "FDA muffed chance to reduce birth defects". Boston Globe. 6 January 2004. Archived from the original on 13 March 2007.
- Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF (September 2002). "Folic acid intake from fortification in United States exceeds predictions". The Journal of Nutrition. 132 (9): 2792–8. doi:10.1093/jn/132.9.2792. PMID 12221247.
- ^ Quinlivan EP, Gregory JF (January 2003). "Effect of food fortification on folic acid intake in the United States". The American Journal of Clinical Nutrition. 77 (1): 221–5. doi:10.1093/ajcn/77.1.221. PMID 12499345.
- "TABLE 1: Nutrient Intakes from Food and Beverages" (PDF). What We Eat In America, NHANES 2012–2014 (2016). Archived (PDF) from the original on 24 February 2017. Retrieved 12 October 2018.
- Centers for Disease Control Prevention (CDC) (16 January 2015). "Updated Estimates of Neural Tube Defects Prevented by Mandatory Folic Acid Fortification — United States, 1995–2011". MMWR. Morbidity and Mortality Weekly Report. 64 (1): 1–5. PMC 4584791. PMID 25590678. Archived from the original on 31 July 2020. Retrieved 15 September 2019.
- "Birth Defects COUNT | Folic Acid | NCBDDD | CDC". www.cdc.gov. Archived from the original on 13 November 2015. Retrieved 16 November 2015.
- ^ Lanska DJ (2009). "Chapter 30 Historical aspects of the major neurological vitamin deficiency disorders: The water-soluble B vitamins". History of Neurology. Handbook of Clinical Neurology. Vol. 95. pp. 445–476. doi:10.1016/S0072-9752(08)02130-1. ISBN 978-0-444-52009-8. PMID 19892133.
- Wills L (May 1978). "Nutrition Classics. British Medical Journal 1:1059–64, 1931. Treatment of "pernicious anaemia of pregnancy" and "tropical anaemia" with special reference to yeast extract as a curative agent. By Lucy Wills". Nutrition Reviews. 36 (5): 149–51. doi:10.1111/j.1753-4887.1978.tb03735.x. PMID 355948.
- Mitchell HK, Snell EE, Williams RJ (1941). "The concentration of "folic acid"". J Am Chem Soc. 63 (8): 2284. doi:10.1021/ja01853a512.
- Paul C (February 2016). "Folic acid in pregnancy". BJOG. 123 (3): 392. doi:10.1111/1471-0528.13602. PMID 26810675.
- Angier RB, Boothe JH, Hutchings BL, Mowat JH, Semb J, Stokstad EL, et al. (August 1945). "Synthesis of a Compound Identical with the L. Casei Factor Isolated from Liver". Science. 102 (2644): 227–8. Bibcode:1945Sci...102..227A. doi:10.1126/science.102.2644.227. PMID 17778509.
- Farber S, Diamond LK (June 1948). "Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid". The New England Journal of Medicine. 238 (23): 787–93. doi:10.1056/NEJM194806032382301. PMID 18860765.
- Forney B (2017). "Folic Acid for Veterinary Use". Wedgewood Pharmacy. Archived from the original on 21 September 2019. Retrieved 21 September 2019.
External links
Current versions from the International Union of Biochemistry and Molecular Biology's Recommendations on Biochemical & Organic Nomenclature, Symbols & Terminology etc. Enzyme Nomenclature, Miscellaneous Reaction Schemes section Pterins, Riboflavins, etc. formerly hosted by Queen Mary College (all archived by archive.org):
- Folate biosynthesis (early stages)
- Folate biosynthesis (later stages)
- Folate coenzymes
- Formylation, hydroxymethylation and methylation using folate
- C1 metabolism with folate
| Related drugs by Anatomical Therapeutic Chemical classification (ATC code) | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
| Dietary supplements | |
|---|---|
| Types | |
| Vitamins and chemical elements ("minerals") | |
| Other common ingredients |
|
| Related articles | |