 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
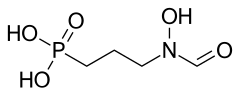
| Formula | C4H10NO5P |
| Molar mass | 183.100 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
Fosmidomycin is an antibiotic that was originally isolated from culture broths of bacteria of the genus Streptomyces. It specifically inhibits DXP reductoisomerase, a key enzyme in the non-mevalonate pathway of isoprenoid biosynthesis. It is a structural analogue of 2-C-methyl-D-erythrose 4-phosphate. It inhibits the E. coli enzyme with a KI value of 38 nM (4), MTB at 80 nM, and the Francisella enzyme at 99 nM. Several mutations in the E. coli DXP reductoisomerase were found to confer resistance to fosmidomycin.
Use in malaria
The discovery of the non-mevalonate pathway in malaria parasites has indicated the use of fosmidomycin and other such inhibitors as antimalarial drugs. Indeed, fosmidomycin has been tested in combination treatment with clindamycin for treatment of malaria with favorable results. It has been shown that an increase in copy number of the target enzyme (DXP reductoisomerase) correlates with in vitro fosmidomycin resistance in the lethal malaria parasite, Plasmodium falciparum.
References
- Iguchi E, Okuhara M, Kohsaka M, Aoki H, Imanaka H (January 1980). "Studies on new phosphonic acid antibiotics. II. Taxonomic studies on producing organisms of the phosphonic acid and related compounds". The Journal of Antibiotics. 33 (1): 19–23. doi:10.7164/antibiotics.33.18. PMID 7372546.
- Jawaid S, Seidle H, Zhou W, Abdirahman H, Abadeer M, Hix JH, van Hoek ML, Couch RD (December 2009). "Kinetic characterization and phosphoregulation of the Francisella tularensis 1-deoxy-D-xylulose 5-phosphate reductoisomerase (MEP synthase)". PLOS ONE. 4 (12): e8288. Bibcode:2009PLoSO...4.8288J. doi:10.1371/journal.pone.0008288. PMC 2788227. PMID 20011597.
- Armstrong CM, Meyers DJ, Imlay LS, Freel Meyers C, Odom AR (September 2015). "Resistance to the antimicrobial agent fosmidomycin and an FR900098 prodrug through mutations in the deoxyxylulose phosphate reductoisomerase gene (dxr)". Antimicrobial Agents and Chemotherapy. 59 (9): 5511–9. doi:10.1128/AAC.00602-15. PMC 4538460. PMID 26124156.
- Pines G, Oh EJ, Bassalo MC, Choudhury A, Garst AD, Fankhauser RG, Eckert CA, Gill RT (November 2018). "Genomic Deoxyxylulose Phosphate Reductoisomerase (DXR) Mutations Conferring Resistance to the Antimalarial Drug Fosmidomycin in E. coli". ACS Synthetic Biology. 7 (12): 2824–2832. doi:10.1021/acssynbio.8b00219. PMC 6928208. PMID 30462485.
- Jomaa H, Wiesner J, Sanderbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenthaler HK, Soldati D, Beck E (September 1999). "Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs". Science. 285 (5433): 1573–6. doi:10.1126/science.285.5433.1573. PMID 10477522.
- Borrmann S, Adegnika AA, Matsiegui PB, Issifou S, Schindler A, Mawili-Mboumba DP, Baranek T, Wiesner J, Jomaa H, Kremsner PG (March 2004). "Fosmidomycin-clindamycin for Plasmodium falciparum Infections in African children". The Journal of Infectious Diseases. 189 (5): 901–8. doi:10.1086/381785. PMID 14976608.
- Borrmann S, Lundgren I, Oyakhirome S, Impouma B, Matsiegui PB, Adegnika AA, Issifou S, Kun JF, Hutchinson D, Wiesner J, Jomaa H, Kremsner PG (August 2006). "Fosmidomycin plus clindamycin for treatment of pediatric patients aged 1 to 14 years with Plasmodium falciparum malaria". Antimicrobial Agents and Chemotherapy. 50 (8): 2713–8. doi:10.1128/AAC.00392-06. PMC 1538678. PMID 16870763.
- Ruangweerayut R, Looareesuwan S, Hutchinson D, Chauemung A, Banmairuroi V, Na-Bangchang K (October 2008). "Assessment of the pharmacokinetics and dynamics of two combination regimens of fosmidomycin-clindamycin in patients with acute uncomplicated falciparum malaria". Malaria Journal. 7 (1): 225. doi:10.1186/1475-2875-7-225. PMC 2600645. PMID 18973702.
- Dharia NV, Sidhu AB, Cassera MB, Westenberger SJ, Bopp SE, Eastman RT, Plouffe D, Batalov S, Park DJ, Volkman SK, Wirth DF, Zhou Y, Fidock DA, Winzeler EA (February 2009). "Use of high-density tiling microarrays to identify mutations globally and elucidate mechanisms of drug resistance in Plasmodium falciparum". Genome Biology. 10 (2): R21. doi:10.1186/gb-2009-10-2-r21. PMC 2688282. PMID 19216790.
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |