| |
| Names | |
|---|---|
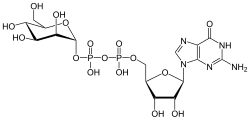
| IUPAC name Guanosine 5′-(α-D-mannopyranosyl dihydrogen diphosphate) | |
| Systematic IUPAC name O-{methyl} O- dihydrogen diphosphate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| MeSH | Guanosine+Diphosphate+Mannose |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H25N5O16P2 |
| Molar mass | 605.341 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases.
Known as donor of activated mannose in all glycolytic reactions, GDP-mannose is essential in eukaryotes.
Biosynthesis
GDP-mannose is produced from GTP and mannose-6-phosphate by the enzyme mannose-1-phosphate guanylyltransferase (GDP-mannose pyrophosphorylase, GDP-MP). This enzyme belongs to a family of nucleotidyl-transferases and is a pervasive enzyme found in bacteria, fungi, plants, and animals.
References
- Stewart, James; Curtis, Joan; Spurck, Timothy P.; Ilg, Thomas; Garami, Attila; Baldwin, Tracey; Courret, Nathalie; McFadden, Geoffrey I.; Davis, Antony; Handman, Emanuela (July 2005). "Characterisation of a Leishmania mexicana knockout lacking guanosine diphosphate-mannose pyrophosphorylase". International Journal for Parasitology. 35 (8): 861–873. doi:10.1016/j.ijpara.2005.03.008. PMID 15936761.
- Samuel G, Reeves P (2003). "Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly". Carbohydrate Research. 338 (23): 2503–19. doi:10.1016/j.carres.2003.07.009. PMID 14670712.
- Pomel, Sébastien; Mao, Wei; Ha-Duong, Tâp; Cavé, Christian; Loiseau, Philippe M. (2019-05-31). "GDP-Mannose Pyrophosphorylase: A Biologically Validated Target for Drug Development Against Leishmaniasis". Frontiers in Cellular and Infection Microbiology. 9: 186. doi:10.3389/fcimb.2019.00186. ISSN 2235-2988. PMC 6554559. PMID 31214516.
See also
| Types of nucleotide sugars | |
|---|---|