| |

| |
| Names | |
|---|---|
| Other names gallium triiodide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.033.269 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | GaI3 |
| Molar mass | 450.436 g/mol |
| Appearance | light yellow powder |
| Density | 4.5 g/cm |
| Melting point | 212 °C (414 °F; 485 K) |
| Boiling point | 340 °C (644 °F; 613 K) |
| Solubility in water | decomposes |
| Magnetic susceptibility (χ) | −149.0·10 cm/mol |
| Thermochemistry | |
| Heat capacity (C) | 100 J/(mol·K) |
| Std molar entropy (S298) |
205.0 J/(mol·K) |
| Std enthalpy of formation (ΔfH298) |
−238.9 kJ/mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H314, H317, H334, H335, H361 |
| Precautionary statements | P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
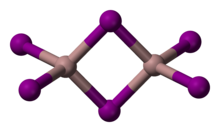
Gallium(III) iodide is the inorganic compound with the formula GaI3. A yellow hygroscopic solid, it is the most common iodide of gallium. In the chemical vapor transport method of growing crystals of gallium arsenide uses iodine as the transport agent. In the solid state, it exists as the dimer Ga2I6. When vaporized, its forms GaI3 molecules of D3h symmetry where the Ga–I distance is 2.458 Angstroms.
Gallium triiodide can be reduced with gallium metal to give a green-colored gallium(I) iodide. The nature of this species is unclear, but it is useful for the preparation of gallium(I) and gallium(II) compounds.
See also
References
- ^ Haynes, p. 4.63
- Haynes, p. 5.20
- Donges, E. (1963). "Gallium(III) Iodide". In Brauer, G. (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1. NY, NY: Academic Press. p. 846.
- Brünig, C.; Locmelis, S.; Milke, E.; Binnewies, M. (2006). "Chemischer Transport fester Lösungen. 27. Mischphasenbildung und chemischer Transport im System Zn Se/Ga As". Zeitschrift für Anorganische und Allgemeine Chemie. 632 (6): 1067–1072. doi:10.1002/zaac.200600008.
- Haynes, p. 9.23
- Baker, Robert J.; Jones, Cameron (2005). ""GaI": A versatile reagent for the synthetic chemist". Dalton Trans (8): 1341–1348. doi:10.1039/b501310k. hdl:2262/69572. PMID 15824768.
- Green, Shaun P.; Jones, Cameron; Stasch, Andreas; Rose, Richard P. (2007). "'GaI': A new reagent for chemo- and diastereoselective C–C bond forming reactions". New J. Chem. 31: 127–134. doi:10.1039/b613669a.
Cited sources
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
| Gallium compounds | |||
|---|---|---|---|
| Gallium(−V) | |||
| Gallium(I) | |||
| Gallium(II) | |||
| Gallium(I,III) | |||
| Gallium(III) |
| ||
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |