
| |
| Names | |
|---|---|
| IUPAC name 1-S--1-thio-beta-D-glucopyranose | |
| Other names Benzyl glucosinolate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 61369 |
| ChEBI | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C14H19NO9S2 |
| Molar mass | 409.42 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Glucotropaeolin or benzyl glucosinolate is a glucosinolate found in cruciferous vegetables, particularly garden cress. Upon enzymatic activity, it is transformed into benzyl isothiocyanate, which contributes to the characteristic flavor of these brassicas.
Occurrence
The compound was first reported in 1899, after its isolation from Tropaeolum majus, a nasturtium species. Glucotropaeolin is now known to occur widely in other brassica families including Caricaceae, Phytolaccaceae, Resedaceae, Salvadoraceae and Tovariaceae.
Structure
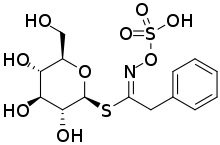
The chemical structure of glucotropaeolin was confirmed by total synthesis in 1957. This showed that it is a glucose derivative with β-D-glucopyranose configuration. At that time it was unclear whether the C=N bond was in the Z form, with sulfur and oxygen substituents on the same side of the double bond, or the alternative E form in which they are on opposite sides. The suggestion was made that the Z form was more likely, based on the known decomposition to benzyl isothiocyanate by a mechanism analogous to the Lossen rearrangement. However, when an identical product was obtained by an alternative route in 1963, it was pointed out that the E form would be expected to rearrange in a similar way. The matter was settled by X-ray crystallography and other spectroscopic studies and it is now known that all natural glucosinolates are of Z form.
Synthesis
Biosynthesis
Glucotropaeolin is biosynthesised from the amino acid phenylalanine in a multi-step pathway.
Laboratory synthesis
The first laboratory syntheses served to confirm the compound's structure. Later work allowed many glucosinolates including this benzyl derivative to be made. These processes are more efficient than isolating pure materials from the plants in which they are naturally found.
Function
Main article: GlucosinolateThe natural role of glucosinolates are as plant defense compounds. The enzyme myrosinase removes the glucose group in glucotropaeolin to give an intermediate which spontaneously rearranges to benzyl isothiocyanate. This is a reactive material which is toxic to many insect predators and its production is triggered when the plant is damaged. This effect has been called the mustard oil bomb. At concentrations typically found in foods, the glucosinolates are not toxic to humans and can be useful flavor components.
References
- "Isothiocyanates". Linus Pauling Institute, Oregon State University.
- ^ Blažević, Ivica; Montaut, Sabine; Burčul, Franko; Olsen, Carl Erik; Burow, Meike; Rollin, Patrick; Agerbirk, Niels (2020). "Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants". Phytochemistry. 169: 112100. doi:10.1016/j.phytochem.2019.112100. PMID 31771793. S2CID 208318505.
- Fahey, Jed W.; Zalcmann, Amy T.; Talalay, Paul (2001). "The chemical diversity and distribution of glucosinolates and isothiocyanates among plants". Phytochemistry. 56 (1): 5–51. doi:10.1016/S0031-9422(00)00316-2. PMID 11198818.
- ^ Ettlinger, Martin G.; Lundeen, Allan J. (1957). "First Synthesis of a Mustard Oil Glucoside; the Enzymatic Lossen Rearrangement". Journal of the American Chemical Society. 79 (7): 1764–1765. doi:10.1021/ja01564a066.
- ^ Benn, M. H. (1963). "A New Mustard Oil Glucoside Synthesis: The Synthesis of Glucotropaeolin". Canadian Journal of Chemistry. 41 (11): 2836–2838. doi:10.1139/v63-415.
- Morant, Anne Vinther; Jørgensen, Kirsten; Jørgensen, Charlotte; Paquette, Suzanne Michelle; Sánchez-Pérez, Raquel; Møller, Birger Lindberg; Bak, Søren (2008). "β-Glucosidases as detonators of plant chemical defense". Phytochemistry. 69 (9): 1795–1813. doi:10.1016/j.phytochem.2008.03.006. PMID 18472115.
- Matile, Ph. (1980). ""Die Senfolbombe": Zur Kompartimentierung des Myrosinasesystems". Biochemie und Physiologie der Pflanzen. 175 (8–9): 722–731. doi:10.1016/S0015-3796(80)80059-X.
- Fenwick, G. Roger; Heaney, Robert K.; Mullin, W. John; Vanetten, Cecil H. (1983). "Glucosinolates and their breakdown products in food and food plants". C R C Critical Reviews in Food Science and Nutrition. 18 (2): 123–201. doi:10.1080/10408398209527361. PMID 6337782.
| Cruciferous biochemistry | |
|---|---|
| Types of compounds | |
| Glucosinolates | |
| Isothiocyanates (ITC, mustard oils) |
|
| Bioactive metabolites | |