| HSD17B1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | HSD17B1, EDH17B2, EDHB17, HSD17, SDR28C1, hydroxysteroid (17-beta) dehydrogenase 1, hydroxysteroid 17-beta dehydrogenase 1, E2DH, 17-beta-HSD, 20-alpha-HSD, Hsd17b1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 109684; MGI: 105077; HomoloGene: 37303; GeneCards: HSD17B1; OMA:HSD17B1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
17β-Hydroxysteroid dehydrogenase 1 (17β-HSD1) is an enzyme that in humans is encoded by the HSD17B1 gene. This enzyme oxidizes or reduces the C17 hydroxy/keto group of androgens and estrogens and hence is able to regulate the potency of these sex steroids
Function
This enzyme is responsible for the interconversion of estrone (E1) and estradiol (E2) and for the interconversion of androstenedione and testosterone:
- 17β-estradiol + NADP + estrone + NADPH + H
- testosterone + NADP + androstenedione + NADPH + H
The human 17β-HSD1 isozyme is highly specific for estrogens over androgens whereas the rodent isozyme is less specific.
Discovery
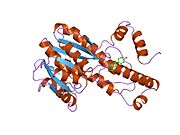
Human 17β-HSD1 was the first enzyme of the 17β-HSD family to be cloned and to have its sequence identified. Its three-dimensional structure is also the first example of any human steroid-converting enzyme.
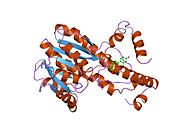
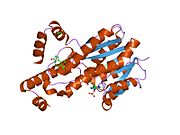
Structure
This enzyme contains a short-chain dehydrogenase domain that contains a characteristic 3-layer (αβα) sandwich known as a Rossmann fold. The human enzyme contains 327 amino acids and exists as a homodimer with two identical subunits of 34.5 kDa The N-terminal short-chain dehydrogenase domain contains binding site for the NADP/NADPH cofactor. A narrow, hydrophobic C-terminal domain contains a binding pocket for the steroid substrate.
Clinical significance
Estradiol stimulates while dihydrotestosterone (DHT) inhibits breast cancer growth. Furthermore 17β-HSD1 levels positively correlate with estradiol and negatively correlate with DHT levels in breast cancer cells. Hence 17β-HSD1 represents a possible drug target for breast cancer treatment.
Inhibitors
See also
Notes
| The 2016 version of this article was updated by an external expert under a dual publication model. The corresponding academic peer reviewed article was published in Gene and can be cited as: Wanhong He, Misra Gauri, Tang Li, Ruixuan Wang, Sheng-Xiang Lin (19 April 2016). "Current knowledge of the multifunctional 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1)". Gene. Gene Wiki Review Series. 588 (1): 54–61. doi:10.1016/J.GENE.2016.04.031. ISSN 0378-1119. PMC 6649686. PMID 27102893. Wikidata Q38814423. |
References
- ^ GRCh38: Ensembl release 89: ENSG00000108786 – Ensembl, May 2017
- ^ GRCm38: Ensembl release 89: ENSMUSG00000019301 – Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Luu-The V, Labrie C, Simard J, Lachance Y, Zhao HF, Couët J, Leblanc G, Labrie F (Feb 1990). "Structure of two in tandem human 17 beta-hydroxysteroid dehydrogenase genes". Molecular Endocrinology. 4 (2): 268–75. doi:10.1210/mend-4-2-268. PMID 2330005.
- Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL, Kedishvili N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U (Mar 2009). "The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative". Chemico-Biological Interactions. 178 (1–3): 94–8. Bibcode:2009CBI...178...94P. doi:10.1016/j.cbi.2008.10.040. PMC 2896744. PMID 19027726.
- "Entrez Gene: HSD17B1 Hydroxysteroid (17-beta) dehydrogenase 1".
- Saloniemi T, Jokela H, Strauss L, Pakarinen P, Poutanen M (2012). "The diversity of sex steroid action: novel functions of hydroxysteroid (17β) dehydrogenases as revealed by genetically modified mouse models". The Journal of Endocrinology. 212 (1): 27–40. doi:10.1530/JOE-11-0315. PMID 22045753.
- Luu The V, Labrie C, Zhao HF, Couët J, Lachance Y, Simard J, Leblanc G, Côté J, Bérubé D, Gagné R (Aug 1989). "Characterization of cDNAs for human estradiol 17 beta-dehydrogenase and assignment of the gene to chromosome 17: evidence of two mRNA species with distinct 5'-termini in human placenta". Molecular Endocrinology. 3 (8): 1301–9. doi:10.1210/mend-3-8-1301. PMID 2779584.
- ^ Peltoketo H, Isomaa V, Mäentausta O, Vihko R (Oct 1988). "Complete amino acid sequence of human placental 17 beta-hydroxysteroid dehydrogenase deduced from cDNA". FEBS Letters. 239 (1): 73–7. doi:10.1016/0014-5793(88)80548-9. PMID 2846351. S2CID 32468574.
- Ghosh D, Pletnev VZ, Zhu DW, Wawrzak Z, Duax WL, Pangborn W, Labrie F, Lin SX (May 1995). "Structure of human estrogenic 17 beta-hydroxysteroid dehydrogenase at 2.20 A resolution". Structure. 3 (5): 503–13. doi:10.1016/S0969-2126(01)00183-6. PMID 7663947.
- Lin SX, Yang F, Jin JZ, Breton R, Zhu DW, Luu-The V, Labrie F (Aug 1992). "Subunit identity of the dimeric 17 beta-hydroxysteroid dehydrogenase from human placenta". The Journal of Biological Chemistry. 267 (23): 16182–7. doi:10.1016/S0021-9258(18)41984-9. PMID 1322895.
- Aka JA, Mazumdar M, Chen CQ, Poirier D, Lin SX (Apr 2010). "17beta-hydroxysteroid dehydrogenase type 1 stimulates breast cancer by dihydrotestosterone inactivation in addition to estradiol production". Molecular Endocrinology. 24 (4): 832–45. doi:10.1210/me.2009-0468. PMC 5417535. PMID 20172961.
- "International Nonproprietary Names for Pharmaceutical Substances (INN) Proposed INN List 130" (PDF). Retrieved 15 August 2024.
Further reading
- Blomquist CH (Dec 1995). "Kinetic analysis of enzymic activities: prediction of multiple forms of 17 beta-hydroxysteroid dehydrogenase". The Journal of Steroid Biochemistry and Molecular Biology. 55 (5–6): 515–24. doi:10.1016/0960-0760(95)00200-6. PMID 8547176. S2CID 21487072.
- Lin SX, Shi R, Qiu W, Azzi A, Zhu DW, Dabbagh HA, Zhou M (Mar 2006). "Structural basis of the multispecificity demonstrated by 17beta-hydroxysteroid dehydrogenase types 1 and 5". Molecular and Cellular Endocrinology. 248 (1–2): 38–46. doi:10.1016/j.mce.2005.11.035. PMID 16480815. S2CID 19087697.
- Peltoketo H, Isomaa V, Vihko R (Oct 1992). "Genomic organization and DNA sequences of human 17 beta-hydroxysteroid dehydrogenase genes and flanking regions. Localization of multiple Alu sequences and putative cis-acting elements". European Journal of Biochemistry. 209 (1): 459–66. doi:10.1111/j.1432-1033.1992.tb17310.x. PMID 1327779.
- Winqvist R, Peltoketo H, Isomaa V, Grzeschik KH, Mannermaa A, Vihko R (Oct 1990). "The gene for 17 beta-hydroxysteroid dehydrogenase maps to human chromosome 17, bands q12-q21, and shows an RFLP with ScaI". Human Genetics. 85 (5): 473–6. doi:10.1007/BF00194219. PMID 1977681. S2CID 38282181.
- Luu-The V, Labrie C, Zhao HF, Couët J, Lachance Y, Simard J, Côté J, Leblanc G, Lagacé L, Bérubé D (1990). "Purification, cloning, complementary DNA structure, and predicted amino acid sequence of human estradiol 17 beta-dehydrogenase". Annals of the New York Academy of Sciences. 595: 40–52. doi:10.1111/j.1749-6632.1990.tb34281.x. PMID 2197970. S2CID 37554424.
- Baker ME (May 1989). "Human placental 17 beta-hydroxysteroid dehydrogenase is homologous to NodG protein of Rhizobium meliloti". Molecular Endocrinology. 3 (5): 881–4. doi:10.1210/mend-3-5-881. PMID 2547159.
- Tremblay Y, Ringler GE, Morel Y, Mohandas TK, Labrie F, Strauss JF, Miller WL (Dec 1989). "Regulation of the gene for estrogenic 17-ketosteroid reductase lying on chromosome 17cen----q25". The Journal of Biological Chemistry. 264 (34): 20458–62. doi:10.1016/S0021-9258(19)47083-X. PMID 2584224.
- Luu The V, Labrie C, Zhao HF, Couët J, Lachance Y, Simard J, Leblanc G, Côté J, Bérubé D, Gagné R (Aug 1989). "Characterization of cDNAs for human estradiol 17 beta-dehydrogenase and assignment of the gene to chromosome 17: evidence of two mRNA species with distinct 5'-termini in human placenta". Molecular Endocrinology. 3 (8): 1301–9. doi:10.1210/mend-3-8-1301. PMID 2779584.
- Peltoketo H, Isomaa V, Mäentausta O, Vihko R (Oct 1988). "Complete amino acid sequence of human placental 17 beta-hydroxysteroid dehydrogenase deduced from cDNA". FEBS Letters. 239 (1): 73–7. doi:10.1016/0014-5793(88)80548-9. PMID 2846351. S2CID 32468574.
- Nicolas JC, Harris JI (Jan 1973). "Human placental 17 -oestradiol dehydrogenase. Sequence of a tryptic peptide containing an essential cysteine". FEBS Letters. 29 (2): 173–6. doi:10.1016/0014-5793(73)80554-X. PMID 4719204. S2CID 44967040.
- Burns DJ, Engel LL, Bethune JL (Jul 1972). "Amino acid composition and subunit structure. Human placental 17 -estradiol dehydrogenase". Biochemistry. 11 (14): 2699–703. doi:10.1021/bi00764a023. PMID 5045524.
- Murdock GL, Chin CC, Offord RE, Bradshaw RA, Warren JC (Oct 1983). "Human placental estradiol 17 beta-dehydrogenase. Identification of a single histidine residue affinity-labeled by both 3-bromoacetoxyestrone and 12 beta-bromoacetoxy-4-estrene-3,17-dione". The Journal of Biological Chemistry. 258 (19): 11460–4. doi:10.1016/S0021-9258(17)44248-7. PMID 6578212.
- Andersson S, Geissler WM, Patel S, Wu L (Jun 1995). "The molecular biology of androgenic 17 beta-hydroxysteroid dehydrogenases". The Journal of Steroid Biochemistry and Molecular Biology. 53 (1–6): 37–9. doi:10.1016/0960-0760(95)00039-3. PMID 7626483. S2CID 54417090.
- Tremblay MR, Auger S, Poirier D (May 1995). "Synthesis of 16-(bromoalkyl)-estradiols having inhibitory effect on human placental estradiol 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD type 1)". Bioorganic & Medicinal Chemistry. 3 (5): 505–23. doi:10.1016/0968-0896(95)00041-E. PMID 7648200.
- Ghosh D, Pletnev VZ, Zhu DW, Wawrzak Z, Duax WL, Pangborn W, Labrie F, Lin SX (May 1995). "Structure of human estrogenic 17 beta-hydroxysteroid dehydrogenase at 2.20 A resolution". Structure. 3 (5): 503–13. doi:10.1016/S0969-2126(01)00183-6. PMID 7663947.
- Sawetawan C, Milewich L, Word RA, Carr BR, Rainey WE (Mar 1994). "Compartmentalization of type I 17 beta-hydroxysteroid oxidoreductase in the human ovary". Molecular and Cellular Endocrinology. 99 (2): 161–8. doi:10.1016/0303-7207(94)90004-3. PMID 8206323. S2CID 54331976.
- Normand T, Narod S, Labrie F, Simard J (Apr 1993). "Detection of polymorphisms in the estradiol 17 beta-hydroxysteroid dehydrogenase II gene at the EDH17B2 locus on 17q11-q21". Human Molecular Genetics. 2 (4): 479–83. doi:10.1093/hmg/2.4.479. PMID 8389226.
- Zhao Z, Yazdani A, Shen Y, Sun Z, Bailey J, Caskey CT, Lee CC (Sep 1996). "Molecular dissection of a cosmid from a gene-rich region in 17q21 and characterization of a candidate gene for alpha-N-acetylglucosaminidase with two cDNA isoforms". Mammalian Genome. 7 (9): 686–90. doi:10.1007/s003359900206. PMID 8703123. S2CID 8692823.