| |
| Names | |
|---|---|
| IUPAC name Hafnium(IV) iodide | |
| Other names hafnium tetraiodide, tetraiodohafnium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.150.349 |
| EC Number |
|
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | HfI4 |
| Molar mass | 686.11 |
| Appearance | red-orange |
| Density | 5.60 g/cm |
| Melting point | 449 °C (840 °F; 722 K) |
| Boiling point | 394 °C (741 °F; 667 K) (sublimes) |
| Structure | |
| Crystal structure | Monoclinic, mS40 |
| Space group | C2/c, No. 15 |
| Lattice constant | a = 1.1787 nm, b = 1.1801 nm, c = 1.2905 nm |
| Related compounds | |
| Other anions | Hafnium(IV) fluoride Hafnium(IV) chloride Hafnium(IV) bromide |
| Other cations | Titanium(IV) iodide Zirconium(IV) iodide |
| Related compounds | Hafnium(III) iodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Hafnium(IV) iodide is the inorganic compound with the formula HfI4. It is a red-orange, moisture sensitive, sublimable solid that is produced by heating a mixture of hafnium with excess iodine. It is an intermediate in the crystal bar process for producing hafnium metal.
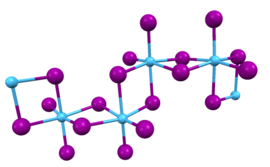
In this compound, the hafnium centers adopt octahedral coordination geometry. Like most binary metal halides, the compound is a polymeric. It is one-dimensional polymer consisting of chains of edge-shared bioctahedral Hf2I8 subunits, similar to the motif adopted by HfCl4. The nonbridging iodide ligands have shorter bonds to Hf than the bridging iodide ligands.
References
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, Florida: CRC Press. p. 4.66. ISBN 1-4398-5511-0.
- ^ Krebs, B.; Sinram, D. (1980). "Hafniumtetrajodid HfI4: Struktur und eigenschaften. Ein neuer AB4-strukturtyp". Journal of the Less Common Metals. 76 (1–2): 7–16. doi:10.1016/0022-5088(80)90005-3.
| Hafnium compounds | |
|---|---|
| Hf(II) | |
| Hf(III) | |
| Hf(IV) | |
| Salts and covalent derivatives of the iodide ion | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||