| This article needs more reliable medical references for verification or relies too heavily on primary sources. Please review the contents of the article and add the appropriate references if you can. Unsourced or poorly sourced material may be challenged and removed. Find sources: "Hemolysis" – news · newspapers · books · scholar · JSTOR (November 2021) |  |
| Hemolysis | |
|---|---|
| Other names | Haemolysis (alternative spelling), hematolysis, erythrolysis, or erythrocytolysis |
 | |
| Specialty | Pathology |
| Complications | Kidney failure, kidney disease |
| Causes | Osmosis |

Hemolysis or haemolysis (/hiːˈmɒlɪsɪs/), also known by several other names, is the rupturing (lysis) of red blood cells (erythrocytes) and the release of their contents (cytoplasm) into surrounding fluid (e.g. blood plasma). Hemolysis may occur in vivo or in vitro.
One cause of hemolysis is the action of hemolysins, toxins that are produced by certain pathogenic bacteria or fungi. Another cause is intense physical exercise. Hemolysins damage the red blood cell's cytoplasmic membrane, causing lysis and eventually cell death.
Etymology
From hemo- + -lysis, from Ancient Greek αἷμα (haîma, 'blood') + λύσις lúsis, 'loosening').
Inside the body
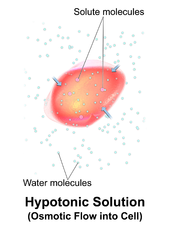
Hemolysis inside the body can be caused by a large number of medical conditions, including some parasites (e.g., Plasmodium), some autoimmune disorders (e.g., autoimmune haemolytic anaemia, drug-induced hemolytic anemia, atypical hemolytic uremic syndrome (aHUS)), some genetic disorders (e.g., Sickle-cell disease or G6PD deficiency), or blood with too low a solute concentration (hypotonic to cells).
Hemolysis can lead to hemoglobinemia due to hemoglobin released into the blood plasma, which plays a significant role in the pathogenesis of sepsis and can lead to increased risk of infection due to its inhibitory effects on the innate immune system.
Parasitic hemolysis
Because the feeding process of the Plasmodium parasites damages red blood cells, malaria is sometimes called "parasitic hemolysis" in medical literature.
HELLP, pre-eclampsia, or eclampsia
- See HELLP syndrome, Pre-eclampsia, and Eclampsia
Hemolytic disease of the newborn
Main article: Hemolytic disease of the newbornHemolytic disease of the newborn is an autoimmune disease resulting from the mother's antibodies crossing the placenta to the fetus. This most often occurs when the mother has previously been exposed to blood antigens present on the fetus but foreign to her, through either a blood transfusion or a previous pregnancy.
Hemolytic anemia
Main article: Hemolytic anemiaBecause in vivo hemolysis destroys red blood cells, in uncontrolled, chronic or severe cases it can lead to hemolytic anemia.
Hemolytic crisis
A hemolytic crisis, or hyperhemolytic crisis, is characterized by an accelerated rate of red blood cell destruction leading to anemia, jaundice, and reticulocytosis. Hemolytic crises are a major concern with sickle-cell disease and G6PD deficiency.
Toxic agent ingestion or poisoning
Paxillus involutus ingestion can cause hemolysis.
Space hemolysis
Spaceflight can cause hemolysis.
Intrinsic causes
Hemolysis may result from intrinsic defects in the red blood cell itself:
- Defects of red blood cell membrane production (as in hereditary spherocytosis and hereditary elliptocytosis)
- Defects in hemoglobin production (as in thalassemia, sickle-cell disease and congenital dyserythropoietic anemia)
- Defective red cell metabolism (as in glucose-6-phosphate dehydrogenase deficiency and pyruvate kinase deficiency)
- Paroxysmal nocturnal hemoglobinuria (PNH), sometimes referred to as Marchiafava-Micheli syndrome, is a rare, acquired, potentially life-threatening disease of the blood characterized by complement-induced intravascular hemolytic anemia.
Extrinsic causes
Extrinsic hemolysis is caused by the red blood cell's environment:
- Immune-mediated causes could include transient factors as in Mycoplasma pneumoniae infection (cold agglutinin disease) or permanent factors as in autoimmune diseases like autoimmune hemolytic anemia (itself more common in diseases such as systemic lupus erythematosus, rheumatoid arthritis, Hodgkin's lymphoma, and chronic lymphocytic leukemia).
- Spur cell hemolytic anemia
- Any of the causes of hypersplenism (increased activity of the spleen), such as portal hypertension.
- Acquired hemolytic anemia is also encountered in burns and as a result of certain infections (e.g. malaria).
- Lead poisoning or poisoning by arsine or stibine causes non-immune hemolytic anemia.
- Runners can develop hemolytic anemia due to "footstrike hemolysis", the destruction of red blood cells in feet at foot impact.
- Low-grade hemolytic anemia occurs in 70% of prosthetic heart valve recipients, and severe hemolytic anemia occurs in 3%.
Intravascular hemolysis
Main article: Intravascular hemolysisIntravascular hemolysis describes hemolysis that happens mainly inside the vasculature. As a result, the contents of the red blood cell are released into the general circulation, leading to hemoglobinemia and increasing the risk of ensuing hyperbilirubinemia.
Intravascular hemolysis may occur when red blood cells are targeted by autoantibodies, leading to complement fixation, or by damage by parasites such as Babesia. Additionally, thrombotic microangiopathy (TMA) can result in hemolysis of red blood cells. TMA is frequently observed in aHUS patients where clots form in the small vessels of the kidney resulting in damaged red blood cells as they attempt to pass through the restricted vessels.
Extravascular hemolysis
Extravascular hemolysis refers to hemolysis taking place in the liver, spleen, bone marrow, and lymph nodes. In this case little hemoglobin escapes into blood plasma. The macrophages of the reticuloendothelial system in these organs engulf and destroy structurally-defective red blood cells, or those with antibodies attached, and release unconjugated bilirubin into the blood plasma circulation. Typically, the spleen destroys mildly abnormal red blood cells or those coated with IgG-type antibodies, while severely abnormal red blood cells or those coated with IgM-type antibodies are destroyed in the circulation or in the liver.
If extravascular hemolysis is extensive, hemosiderin can be deposited in the spleen, bone marrow, kidney, liver, and other organs, resulting in hemosiderosis.
Outside the body

In vitro hemolysis can be caused by improper technique during collection of blood specimens, by the effects of mechanical processing of blood, or by bacterial action in cultured blood specimens.
From specimen collection
Most causes of in vitro hemolysis are related to specimen collection. Difficult collections, unsecure line connections, contamination, and incorrect needle size, as well as improper tube mixing and incorrectly filled tubes are all frequent causes of hemolysis. Excessive suction can cause the red blood cells to be smashed on their way through the hypodermic needle owing to turbulence and physical forces. Such hemolysis is more likely to occur when a patient's veins are difficult to find or when they collapse when blood is removed by a syringe or a modern vacuum tube. Experience and proper technique are key for any phlebotomist, nurse or doctor to prevent hemolysis.
In vitro hemolysis during specimen collection can cause inaccurate laboratory test results by contaminating the surrounding plasma with the contents of hemolyzed red blood cells. For example, the concentration of potassium inside red blood cells is much higher than in the plasma and so an elevated potassium level is usually found in biochemistry tests of hemolyzed blood.
After the blood collection process, in vitro hemolysis can still occur in a sample due to external factors, such as prolonged storage, incorrect storage conditions and excessive physical forces by dropping or vigorously mixing the tube.
From mechanical blood processing during surgery
In some surgical procedures (especially some heart operations) where substantial blood loss is expected, machinery is used for intraoperative blood salvage. A centrifuge process takes blood from the patient, washes the red blood cells with normal saline, and returns them to the patient's blood circulation. Hemolysis may occur if the centrifuge rotates too quickly (generally greater than 500 rpm)—essentially this is hemolysis occurring outside of the body. Increased hemolysis occurs with massive amounts of sudden blood loss, because the process of returning a patient's cells must be done at a correspondingly higher speed to prevent hypotension, pH imbalance, and a number of other hemodynamic and blood level factors. Modeling of fluid flows to predict the likelihood of red cell membrane rupture in response to stress is an active area of research.
From bacteria culture
Main article: Hemolysis (microbiology)
Visualizing the physical appearance of hemolysis in cultured blood samples may be used as a tool to determine the species of various Gram-positive bacteria infections (e.g., Streptococcus).
Nomenclature
Hemolysis is sometimes called hematolysis, erythrolysis, or erythrocytolysis. The words hemolysis (/hiːˈmɒlɪsɪs/) and hematolysis (/ˌhiːməˈtɒlɪsɪs/) both use combining forms conveying the idea of "lysis of blood" (hemo- or hemato- + -lysis). The words erythrolysis (/ˌɛrəˈθrɒlɪsɪs/) and erythrocytolysis (/əˌrɪθroʊsaɪˈtɒlɪsɪs/) both use combining forms conveying the idea of "lysis of erythrocytes" (erythro- ± cyto- + -lysis).
Red blood cells (erythrocytes) have a short lifespan (approximately 120 days), and old (senescent) cells are constantly removed and replaced with new ones via erythropoiesis. This breakdown/replacement process is called erythrocyte turnover. In this sense, erythrolysis or hemolysis is a normal process that happens continually. However, these terms are usually used to indicate that the lysis is pathological.
Complications
See also: Hemolytic anemia § Signs and symptomsPulmonary hypertension has been gaining recognition as a complication of chronic hereditary and acquired hemolysis. Free hemoglobin released during hemolysis inactivates the vasodilator nitric oxide (NO). Hemolysis also releases arginase that depletes L-arginine, the substrate needed for NO synthesis. This reduces NO-dependent vasodilation and induces platelet activation, thrombin generation, procoagulant factors and tissue factor activation, contributing to the formation of thrombosis. This can lead to esophageal spasm and dysphagia, abdominal pain, erectile dysfunction, systemic hypertension, decreased organ perfusion, promotion of inflammation and coagulation, and thrombosis.
Chronic hemolysis may also lead to endothelial dysfunction, heightened endothelin-1-mediated responses and vasculopathy. The release of heme leads to the production of bilirubin and depletion of plasma proteins, such as albumin, haptoglobin, and hemopexin, which may lead to jaundice. It may also lead to increased levels of the heme breakdown product stercobilin in the stool.
Splenectomy of those with hemolytic disorders appears to increase risk of developing pulmonary thrombosis.
Complications may also arise from the increased workload for the kidney as it secretes erythropoietin to stimulate the bone marrow to produce more reticulocytes (red blood cell precursors) to compensate for the loss of red blood cells due to hemolysis.
See also
References
- ^ Wells, John C. (2008). Longman Pronunciation Dictionary (3rd ed.). Longman. ISBN 978-1-4058-8118-0.
- Witek, K; Ścisłowska, J; Turowski, D; Lerczak, K; Lewandowska-Pachecka, S; Pokrywka, A (March 2017). "Total bilirubin in athletes, determination of reference range". Biology of Sport. 34 (1): 45–48. doi:10.5114/biolsport.2017.63732. ISSN 0860-021X. PMC 5377560. PMID 28416897.
- Madigan, Michael T. (2010). Brock Biology of Microorganisms 13th Edition. Benjamin Cummings. p. 804. ISBN 978-0-321-64963-8.
- "Atypical hemolytic-uremic syndrome". Genetics Home Reference. Retrieved 2020-08-18.
- ^ Barcellini, Wilma (2015). "Immune Hemolysis: Diagnosis and Treatment Recommendations". Seminars in Hematology. 52 (4): 304–312. doi:10.1053/j.seminhematol.2015.05.001. ISSN 1532-8686. PMID 26404442.
- ^ Beris, Photis; Picard, Véronique (2015). "Non-immune Hemolysis: Diagnostic Considerations". Seminars in Hematology. 52 (4): 287–303. doi:10.1053/j.seminhematol.2015.07.005. ISSN 1532-8686. PMID 26404441.
- ^ Effenberger-Neidnicht, Katharina; Hartmann, Matthias (2018). "Mechanisms of Hemolysis During Sepsis". Inflammation. 41 (5): 1569–1581. doi:10.1007/s10753-018-0810-y. ISSN 1573-2576. PMID 29956069. S2CID 49526899.
- "Erythrocyte Alloimmunization and Pregnancy: Overview, Background, Pathophysiology". 2019-02-02.
{{cite journal}}: Cite journal requires|journal=(help) - "Innvista". Archived from the original on 2010-06-24. Retrieved 2010-07-04.
- Guzman, Ana (2022-02-24). "Scientists Find Increased Red Blood Cell Destruction in Space". NASA. Retrieved 2022-06-09.
- Jacobasch, G; Rapoport, SM (1996). "Hemolytic anemias due to erythrocyte enzyme deficiencies". Molecular Aspects of Medicine. 17 (2): 143–70. doi:10.1016/0098-2997(96)88345-2. ISSN 0098-2997. PMID 8813716.
- Bossi, D; Russo, M (1996). "Hemolytic anemias due to disorders of red cell membrane skeleton". Molecular Aspects of Medicine. 17 (2): 171–88. doi:10.1016/0098-2997(96)88346-4. ISSN 0098-2997. PMID 8813717.
- Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, Smith JA (January 2003). "Footstrike is the major cause of hemolysis during running". J. Appl. Physiol. 94 (1): 38–42. doi:10.1152/japplphysiol.00631.2001. PMID 12391035. S2CID 5750453.
- Lippi G, Schena F, Salvagno GL, Aloe R, Banfi G, Guidi GC (July 2012). "Foot-strike haemolysis after a 60-km ultramarathon". Blood Transfus. 10 (3): 377–383. doi:10.2450/2012.0167-11. PMC 3417738. PMID 22682343.
- Wise, Donald Lee (2000). Biomaterials Engineering and Devices: Orthopedic, dental, and bone graft applications. Humana Press. ISBN 978-0-89603-859-2.
- ^ Stanley L Schrier. William C Mentzer; Jennifer S Tirnauer (eds.). "Diagnosis of hemolytic anemia in the adult". UpToDate. Archived from the original on 2017-12-26. Retrieved 2019-05-04.
- "Intravascular hemolysis". eClinpath. Retrieved 2019-05-08.
- ^ Muller, Andre; Jacobsen, Helene; Healy, Edel; McMickan, Sinead; Istace, Fréderique; Blaude, Marie-Noëlle; Howden, Peter; Fleig, Helmut; Schulte, Agnes (2006). "Hazard classification of chemicals inducing haemolytic anaemia: An EU regulatory perspective" (PDF). Regulatory Toxicology and Pharmacology. 45 (3). Elsevier BV: 229–241. doi:10.1016/j.yrtph.2006.04.004. hdl:10029/5596. ISSN 0273-2300. PMID 16793184. Archived from the original (PDF) on 2019-05-03. Retrieved 2019-05-04.
- "Bilirubin and hemolytic anemia". eClinpath. Retrieved 2019-05-08.
- "Thrombotic Microangiopathy (TMA)". UNC Kidney Center. Retrieved 2020-08-19.
- "Atypical Hemolytic Uremic Syndrome". NORD (National Organization for Rare Disorders). Retrieved 2020-08-19.
- Rhodes, Carl E.; Varacallo, Matthew (2019-03-04). "Physiology, Oxygen Transport". NCBI Bookshelf. PMID 30855920. Retrieved 2019-05-04.
- Sokol RJ, Hewitt S, Stamps BK (June 1981). "Autoimmune haemolysis: an 18-year study of 865 cases referred to a regional transfusion centre". Br Med J (Clin Res Ed). 282 (6281): 2023–7. doi:10.1136/bmj.282.6281.2023. PMC 1505955. PMID 6788179.
- ^ Braunstein, Evan (2019-05-03). "Overview of Hemolytic Anemia – Hematology and Oncology". Merck Manuals Professional Edition (in Latin). Retrieved 2019-05-05.
- "Hypersplenism: MedlinePlus Medical Encyclopedia". MedlinePlus. 2019-04-30. Retrieved 2019-05-08.
- "Capital Health".
- McCaughey, Euan James; Vecellio, Elia; Lake, Rebecca; Li, Ling; Burnett, Leslie; Chesher, Douglas; Braye, Stephen; Mackay, Mark; Gay, Stephanie (2017-01-02). "Key factors influencing the incidence of hemolysis: A critical appraisal of current evidence". Critical Reviews in Clinical Laboratory Sciences. 54 (1): 59–72. doi:10.1080/10408363.2016.1250247. ISSN 1040-8363. PMID 28013559. S2CID 753640.
- Faghih, Mohammad M.; Sharp, M. Keith (2019-03-07). "Modeling and prediction of flow-induced hemolysis: a review". Biomechanics and Modeling in Mechanobiology. 18 (4): 845–881. doi:10.1007/s10237-019-01137-1. ISSN 1617-7940. PMID 30847662. S2CID 73496695.
- "hematolysis". Merriam-Webster.com Dictionary. Merriam-Webster. Retrieved 8 July 2018.
- "erythrolysis". American Heritage Stedman's Medical Dictionary. Houghton Mifflin. Archived from the original on 8 July 2018. Retrieved 8 July 2018.
- "erythrocytolysis". American Heritage Stedman's Medical Dictionary. Houghton Mifflin. Archived from the original on 8 July 2018. Retrieved 8 July 2018.
- ^ Machado, Roberto F.; Gladwin, Mark T. (2010). "Pulmonary Hypertension in Hemolytic Disorders". Chest. 137 (6). Elsevier BV: 30S–38S. doi:10.1378/chest.09-3057. ISSN 0012-3692. PMC 2882115. PMID 20522578.
- Rother, Russell P.; Bell, Leonard; Hillmen, Peter; Gladwin, Mark T. (2005-04-06). "The Clinical Sequelae of Intravascular Hemolysis and Extracellular Plasma Hemoglobin". JAMA. 293 (13). American Medical Association (AMA): 1653–62. doi:10.1001/jama.293.13.1653. ISSN 0098-7484. PMID 15811985.
- ^ Reiter, Christopher D.; Wang, Xunde; Tanus-Santos, Jose E.; Hogg, Neil; Cannon, Richard O.; Schechter, Alan N.; Gladwin, Mark T. (2002-11-11). "Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease". Nature Medicine. 8 (12). Springer Nature: 1383–1389. doi:10.1038/nm1202-799. ISSN 1078-8956. PMID 12426562. S2CID 19878520.
- Rother, Russell P.; Bell, Leonard; Hillmen, Peter; Gladwin, Mark T. (2005-04-06). "The Clinical Sequelae of Intravascular Hemolysis and Extracellular Plasma Hemoglobin". JAMA. 293 (13): 1653–1662. doi:10.1001/jama.293.13.1653. ISSN 0098-7484. PMID 15811985.
The systemic removal of nitric oxide has been shown to contribute to clinical morbidities, including severe esophageal spasm and dysphagia, abdominal pain, erectile dysfunction, and thrombosis.16,17,23-26 In addition, systemic release of hemoglobin is associated with pulmonary and systemic hypertension,17,20,53-55 decreased organ perfusion, and increased mortality.53-58 Plasma hemoglobin and its breakdown product heme can also directly activate endothelial cells and further promote inflammation and coagulation.27
- Schaer, D. J.; Buehler, P. W.; Alayash, A. I.; Belcher, J. D.; Vercellotti, G. M. (2012-12-20). "Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins". Blood. 121 (8). American Society of Hematology: 1276–1284. doi:10.1182/blood-2012-11-451229. ISSN 0006-4971. PMC 3578950. PMID 23264591.
- Smith, Ann; McCulloh, Russell J. (2015-06-30). "Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders". Frontiers in Physiology. 6. Frontiers Media SA: 187. doi:10.3389/fphys.2015.00187. ISSN 1664-042X. PMC 4485156. PMID 26175690.
- Schaer, Dominik J.; Vinchi, Francesca; Ingoglia, Giada; Tolosano, Emanuela; Buehler, Paul W. (2014-10-28). "Haptoglobin, hemopexin, and related defense pathways—basic science, clinical perspectives, and drug development". Frontiers in Physiology. 5. Frontiers Media SA: 415. doi:10.3389/fphys.2014.00415. ISSN 1664-042X. PMC 4211382. PMID 25389409.
 Material was copied from this source, which is available under a Creative Commons License.
Material was copied from this source, which is available under a Creative Commons License.
External links
| Classification | D |
|---|