| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,3,5,7-Tetraazaadamantane | |||
Other names
| |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 2018 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.642 | ||
| EC Number |
| ||
| E number | E239 (preservatives) | ||
| Gmelin Reference | 26964 | ||
| KEGG | |||
| MeSH | Methenamine | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1328 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H12N4 | ||
| Molar mass | 140.186 g/mol | ||
| Appearance | White crystalline solid | ||
| Odor | Fishy, ammonia like | ||
| Density | 1.33 g/cm (at 20 °C) | ||
| Melting point | 280 °C (536 °F; 553 K) (sublimes) | ||
| Solubility in water | 85.3 g/100 mL | ||
| Solubility | Soluble in chloroform, methanol, ethanol, acetone, benzene, xylene, ether | ||
| Solubility in chloroform | 13.4 g/100 g (20 °C) | ||
| Solubility in methanol | 7.25 g/100 g (20 °C) | ||
| Solubility in ethanol | 2.89 g/100 g (20 °C) | ||
| Solubility in acetone | 0.65 g/100 g (20 °C) | ||
| Solubility in benzene | 0.23 g/100 g (20 °C) | ||
| Acidity (pKa) | 4.89 | ||
| Pharmacology | |||
| ATC code | J01XX05 (WHO) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Highly combustible, harmful | ||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Warning | ||
| Hazard statements | H228, H317 | ||
| Precautionary statements | P210, P240, P241, P261, P272, P280, P302+P352, P321, P333+P313, P363, P370+P378, P501 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 250 °C (482 °F; 523 K) | ||
| Autoignition temperature |
410 °C (770 °F; 683 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
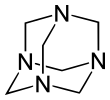
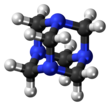
Hexamethylenetetramine (HMTA), also known as 1,3,5,7-tetraazaadamantane, is a heterocyclic organic compound with diverse applications. It has the chemical formula (CH2)6N4 and is a white crystalline compound that is highly water soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other organic compounds, including plastics, pharmaceuticals, and rubber additives. The compound is also used medically for certain conditions. It sublimes in vacuum at 280 °C. The molecule has a tetrahedral cage-like structure, similar to that of adamantane. Four vertices are occupied by nitrogen atoms, which are linked by methylene groups. Although the molecular shape defines a cage, no void space is available at the interior.
Synthesis, structure, reactivity
Hexamethylenetetramine was discovered by Aleksandr Butlerov in 1859. It is prepared industrially by combining formaldehyde and ammonia:
The molecule behaves like an amine base, undergoing protonation and as a ligand. N-alkylation with chloroallyl chloride gives quaternium-15).
Applications
The dominant use of hexamethylenetetramine is in the production of solid (powder) or liquid phenolic resins and phenolic resin moulding compounds, in which it is added as a hardening component. These products are used as binders, e.g., in brake and clutch linings, abrasives, non-woven textiles, formed parts produced by moulding processes, and fireproof materials.
Medical uses
Main article: Methenamine (drug)The compound is also used medically as a urinary antiseptic and antibacterial medication under the name methenamine or hexamine. It is used as an alternative to antibiotics to prevent urinary tract infections (UTIs) and is sold under the brand names Hiprex, Urex, and Urotropin, among others.
As the mandelic acid salt (methenamine mandelate) or the hippuric acid salt (methenamine hippurate), it is used for the treatment of urinary tract infections. In an acidic environment, methenamine is believed to act as an antimicrobial by converting to formaldehyde. A systematic review of its use for this purpose in adult women found there was insufficient evidence of benefit and further research was needed. A UK study showed that methenamine is as effective as daily low-dose antibiotics at preventing UTIs among women who experience recurrent UTIs. As methenamine is an antiseptic, it may avoid the issue of antibiotic resistance.
Methenamine acts as an over-the-counter antiperspirant due to the astringent property of formaldehyde. Specifically, methenamine is used to minimize perspiration in the sockets of prosthetic devices.
Histological stains
Methenamine silver stains are used for staining in histology, including the following types:
- Grocott's methenamine silver stain, used widely as a screen for fungal organisms.
- Jones' stain, a methenamine silver-Periodic acid-Schiff that stains for basement membrane, availing to view the "spiked" Glomerular basement membrane associated with membranous glomerulonephritis.
Solid fuel
Together with 1,3,5-trioxane, hexamethylenetetramine is a component of hexamine fuel tablets used by campers, hobbyists, the military and relief organizations for heating camping food or military rations. It burns smokelessly, has a high energy density of 30.0 megajoules per kilogram (MJ/kg), does not liquify while burning, and leaves no ashes, although its fumes are toxic.
Standardized 0.149 g tablets of methenamine (hexamine) are used by fire-protection laboratories as a clean and reproducible fire source to test the flammability of carpets and rugs.
Food additive
Hexamethylenetetramine or hexamine is also used as a food additive as a preservative (INS number 239). It is approved for usage for this purpose in the EU, where it is listed under E number E239, however it is not approved in the USA, Russia, Australia, or New Zealand.
Reagent in organic chemistry
Hexamethylenetetramine is a versatile reagent in organic synthesis. It is used in the Duff reaction (formylation of arenes), the Sommelet reaction (converting benzyl halides to aldehydes), and in the Delepine reaction (synthesis of amines from alkyl halides).
Explosives
Hexamethylenetetramine is the base component to produce RDX and, consequently, C-4 as well as octogen (a co-product with RDX), hexamine dinitrate, hexamine diperchlorate and HMTD.
Pyrotechnics
Hexamethylenetetramine is also used in pyrotechnics to reduce combustion temperatures and decrease the color intensity of various fireworks. Because of its ash-free combustion, hexamethylenetetramine is also utilized in indoor fireworks alongside magnesium and lithium salts.
Historical uses
Hexamethylenetetramine was first introduced into the medical setting in 1895 as a urinary antiseptic. It was officially approved by the FDA for medical use in the United States in 1967. However, it was only used in cases of acidic urine, whereas boric acid was used to treat urinary tract infections with alkaline urine. Scientist De Eds found that there was a direct correlation between the acidity of hexamethylenetetramine's environment and the rate of its decomposition. Therefore, its effectiveness as a drug depended greatly on the acidity of the urine rather than the amount of the drug administered. In an alkaline environment, hexamethylenetetramine was found to be almost completely inactive.
Hexamethylenetetramine was also used as a method of treatment for soldiers exposed to phosgene in World War I. Subsequent studies have shown that large doses of hexamethylenetetramine provide some protection if taken before phosgene exposure but none if taken afterwards.

Producers
Since 1990 the number of European producers has been declining. The French SNPE factory closed in 1990; in 1993, the production of hexamethylenetetramine in Leuna, Germany ceased; in 1996, the Italian facility of Agrolinz closed down; in 2001, the UK producer Borden closed; in 2006, production at Chemko, Slovak Republic, was closed. Remaining producers include INEOS in Germany, Caldic in the Netherlands, and Hexion in Italy. In the US, Eli Lilly and Company stopped producing methenamine tablets in 2002. In Australia, Hexamine Tablets for fuel are made by Thales Australia Ltd. In México, Hexamine is produced by Abiya. Many other countries who still produce this include Russia, Saudi Arabia, and China.
References
- Cooney AP, Crampton MR, Golding P (1986). "The acid-base behaviour of hexamine and its N-acetyl derivatives". J. Chem. Soc., Perkin Trans. 2 (6): 835–839. doi:10.1039/P29860000835.
- ^ Dreyfors JM, Jones SB, Sayed Y (November 1989). "Hexamethylenetetramine: a review". American Industrial Hygiene Association Journal. 50 (11): 579–585. doi:10.1080/15298668991375191. PMID 2688388.
- ^ Blažzević, Nikola; Kolbah, D.; Belin, Branka; Šunjić, Vitomir; Kajfež, Franjo (1979). "Hexamethylenetetramine, A Versatile Reagent in Organic Synthesis". Synthesis. 1979 (3). Georg Thieme Verlag KG: 161–176. doi:10.1055/s-1979-28602. ISSN 0039-7881.
- ^ Lo TS, Hammer KD, Zegarra M, Cho WC (May 2014). "Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era". Expert Rev Anti Infect Ther. 12 (5): 549–554. doi:10.1586/14787210.2014.904202. PMID 24689705.
- Connolly M, de Berker D (2003). "Management of primary hyperhidrosis: a summary of the different treatment modalities". Am J Clin Dermatol. 4 (10): 681–697. doi:10.2165/00128071-200304100-00003. PMID 14507230.
- Butlerow A (1859). "Ueber einige Derivate des Jodmethylens" [On some derivatives of methylene iodide]. Ann. Chem. Pharm. (in German). 111 (2): 242–252. doi:10.1002/jlac.18591110219. In this article, Butlerov discovered formaldehyde, which he called "dioxymethylen" (methylene dioxide) because his empirical formula for it was incorrect (C4H4O4). On pages 249–250, he describes treating formaldehyde with ammonia gas, creating hexamine.
- Butlerow A (1860). "Ueber ein neues Methylenderivat" [On a new methylene derivative]. Ann. Chem. Pharm. (in German). 115 (3): 322–327. doi:10.1002/jlac.18601150325.
- ^ Eller K, Henkes E, Rossbacher R, Höke H (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH. doi:10.1002/14356007.a02_001. ISBN 9783527306732.
- Kirillov, Alexander M. (2011). "Hexamethylenetetramine: An old new building block for design of coordination polymers". Coordination Chemistry Reviews. 255 (15–16). Elsevier BV: 1603–1622. doi:10.1016/j.ccr.2011.01.023. ISSN 0010-8545.
- ^ https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/017681Orig1s019lbl.pdf
- Elks, J. (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 629. ISBN 978-1-4757-2085-3. Retrieved 11 October 2024.
- ^ Schweizerischer Apotheker-Verein (2004). Index Nominum: International Drug Directory. Medpharm Scientific Publishers. pp. 775–776. ISBN 978-3-88763-101-7. Retrieved 11 October 2024.
- ^ Lo TS, Hammer KD, Zegarra M, Cho WC (May 2014). "Methenamine: a forgotten drug for preventing recurrent urinary tract infection in a multidrug resistance era". Expert Review of Anti-Infective Therapy. 12 (5): 549–554. doi:10.1586/14787210.2014.904202. PMID 24689705. S2CID 207199202.
- Chwa A, Kavanagh K, Linnebur SA, Fixen DR (2019). "Evaluation of methenamine for urinary tract infection prevention in older adults: a review of the evidence". Therapeutic Advances in Drug Safety. 10: 2042098619876749. doi:10.1177/2042098619876749. PMC 6759703. PMID 31579504.
- Bakhit M, Krzyzaniak N, Hilder J, Clark J, Scott AM, Mar CD (July 2021). "Use of methenamine hippurate to prevent urinary tract infections in community adult women: a systematic review and meta-analysis". The British Journal of General Practice (Systematic review). 71 (708): e528–e537. doi:10.3399/BJGP.2020.0833. PMC 8136580. PMID 34001538.
- "Methenamine is as good as antibiotics at preventing urinary tract infections". NIHR Evidence. 2022-12-20. doi:10.3310/nihrevidence_55378. S2CID 254965605.
- Harding C, Mossop H, Homer T, Chadwick T, King W, Carnell S, et al. (March 2022). "Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, non-inferiority trial". BMJ. 376: e068229. doi:10.1136/bmj-2021-0068229. PMC 8905684. PMID 35264408.
- Susak Z, Minkov R, Isakov E (December 1996). "The use of Methenamine as an antiperspirant for amputees". Prosthetics and Orthotics International. 20 (3): 172–175. doi:10.3109/03093649609164439. PMID 8985996. S2CID 24088433.
- Susak, Z.; Minkov, R.; Isakov, E. (December 1996). "The use of Methenamine as an antiperspirant for amputees". Prosthetics & Orthotics International. 20 (3): 172–175. doi:10.3109/03093649609164439. ISSN 0309-3646. PMID 8985996. S2CID 24088433.
- ^ "Re: Equialence of methenamine Tablets Standard for Flammability of Carpets and Rugs" (PDF). Alan H. Schoen. July 29, 2004. Archived from the original (PDF) on 2008-10-05.
- UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". 8 September 2011. Retrieved 2011-10-27.
- Blažzević N, Kolbah D, Belin B, Šunjić V, Kajfež F (1979). "Hexamethylenetetramine, A Versatile Reagent in Organic Synthesis". Synthesis. 1979 (3): 161–176. doi:10.1055/s-1979-28602. S2CID 97756228.
- Allen CH, Leubne GW (1951). "Syringic Aldehyde". Organic Syntheses. 31: 92. doi:10.15227/orgsyn.031.0092.
- Wiberg KB (1963). "2-Thiophenaldehyde". Organic Syntheses. doi:10.15227/orgsyn.000.0000; Collected Volumes, vol. 3, p. 811.
- Bottini AT, Dev V, Klinck J (1963). "2-Bromoallylamine". Organic Syntheses. 43: 6. doi:10.15227/orgsyn.043.0006.
- DE patent 3402546, Lohmann, Erwin, "Pyrotechnischer Satz zur Erzeugung von Lichtblitzen", issued 1985-08-01
- US patent 6214139, Hiskey, Michael A.; Chavez, David E. & Naud, Darren L., "Low-smoke pyrotechnic compositions", issued 2001-04-10, assigned to The Regents of the University of California and Los Alamos National Laboratory
- GB patent 2502460, Wraige, John Douglas Michael, "Pyrotechnic composition with spark producing material", issued 2013-11-27
- Hinman F (1913). "An Experimental Study of the Antiseptic Value in the Urine of the Internal Use of Hexamethylenamin". JAMA: The Journal of the American Medical Association. 61 (18): 1601. doi:10.1001/jama.1913.04350190019006.
- Sauberan, Jason B.; Bradley, John S. (2018-01-01), Long, Sarah S.; Prober, Charles G.; Fischer, Marc (eds.), "292 - Antimicrobial Agents", Principles and Practice of Pediatric Infectious Diseases (Fifth Edition), Elsevier, pp. 1499–1531.e3, doi:10.1016/b978-0-323-40181-4.00292-9, ISBN 978-0-323-40181-4, retrieved 2023-11-15
- ^ Elliot (1913). "On Urinary Antiseptics". British Medical Journal. 98: 685–686.
- Heathcote RS (1935). "Hexamine as an Urinary Antiseptic: I. Its Rate of Hydrolysis at Different Hydrogen Ion Concentrations. II. Its Antiseptic Power Against Various Bacteria in Urine". British Journal of Urology. 7 (1): 9–32. doi:10.1111/j.1464-410X.1935.tb11265.x. ISSN 0007-1331.
- Diller WF (December 1980). "The methenamine misunderstanding in the therapy of phosgene poisoning". Archives of Toxicology. 46 (3–4): 199–206. Bibcode:1980ArTox..46..199D. doi:10.1007/BF00310435. PMID 7016075. S2CID 2423812.
| Antibacterials: others (J01X, D06AX) | |
|---|---|
| Glycopeptides Lipoglycopeptides | |
| Polymyxins | |
| Steroid antibacterials | |
| Imidazole derivatives | |
| Pleuromutilins | |
| Nitrofuran derivatives | |
| Other antibacterials | |