| |

| |
| Names | |
|---|---|
| IUPAC name Hexaamminecobalt(III) chloride | |
| Other names Cobalt hexammine chloride, hexaamminecobalt(III) chloride | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.991 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | H18N6Cl3Co |
| Molar mass | 267.48 g/mol |
| Appearance | yellow or orange crystals |
| Density | 1.71 g/cm, |
| Melting point | decomposes |
| Solubility in water | 0.26 M (20 °C) tribromide: 0.04 M (18 °C) |
| Solubility | soluble in NH3 |
| Structure | |
| Coordination geometry | octahedral |
| Dipole moment | 0 D |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | poison |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Related compounds | |
| Other anions | Br3 (OAc)3 |
| Other cations | Cl3 Cl2 |
| Related compounds | Cl3 Cl3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
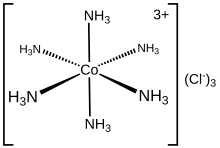
Hexaamminecobalt(III) chloride is the chemical compound with the formula Cl3. It is the chloride salt of the coordination complex , which is considered an archetypal "Werner complex", named after the pioneer of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion.
Properties and structure
is diamagnetic, with a low-spin 3d octahedral Co(III) center. The cation obeys the 18-electron rule and is considered to be a classic example of an exchange inert metal complex. As a manifestation of its inertness, Cl3 can be recrystallized unchanged from concentrated hydrochloric acid: the NH3 is so tightly bound to the Co(III) centers that it does not dissociate to allow its protonation. In contrast, labile metal ammine complexes, such as Cl2, react rapidly with acids, reflecting the lability of the Ni(II)–NH3 bonds. Upon heating, hexamminecobalt(III) begins to lose some of its ammine ligands, eventually producing a stronger oxidant.
The chloride ions in Cl3 can be exchanged with a variety of other anions such as nitrate, bromide, iodide, sulfamate to afford the corresponding X3 derivative. Such salts are orange or bright yellow and display varying degrees of water solubility. The chloride ion can be also exchanged with more complex anions such as the hexathiocyanatochromate(III), yielding a pink compound with formula , or the ferricyanide ion.
Preparation
Cl3 is prepared by treating cobalt(II) chloride with ammonia and ammonium chloride followed by oxidation. Oxidants include hydrogen peroxide or oxygen in the presence of charcoal catalyst. This salt appears to have been first reported by Fremy.
The acetate salt can be prepared by aerobic oxidation of cobalt(II) acetate, ammonium acetate, and ammonia in methanol. The acetate salt is highly water-soluble to the level of 1.9 M (20 °C), versus 0.26 M for the trichloride.
Uses in the laboratory
is a component of some structural biology methods (especially for DNA or RNA, where positive ions stabilize tertiary structure of the phosphate backbone), to help solve their structures by X-ray crystallography or by nuclear magnetic resonance. In the biological system, the counterions would more probably be Mg, but the heavy atoms of cobalt (or sometimes iridium, as in PDB: 2GIS) provide anomalous scattering to solve the phase problem and produce an electron-density map of the structure.
is used to investigate DNA. The cation induces the transition of DNA structure from the classical B-form to the Z-form.
Related compounds
References
- ^ Bjerrum, J.; McReynolds, J. P. (1946). "Hexamminecobalt(III) Salts". Inorg. Synth. 2: 216–221. doi:10.1002/9780470132333.ch69.
- Fremy, M. E. (1852). "Recherches sur le cobalt". Ann. Chim. Phys. 35: 257–312.
- Lindholm, R. D.; Bause, Daniel E. (1978). "Complexes of Cobalt Containing Ammonia or Ethylene Diamine: Hexaamminecobalt(III) Salts". Inorg. Synth. 18: 67–69. doi:10.1002/9780470132494.ch14.
- Ramakrishnan, B.; Sekharudu, C.; Pan, B.; Sundaralingam, M. (2003). "Near-atomic resolution crystal structure of an A-DNA decamer d(CCCGATCGGG): cobalt hexammine interaction with A-DNA". Acta Crystallogr. D59 (Pt 1): 67–72. Bibcode:2003AcCrD..59...67R. doi:10.1107/s0907444902018917. PMID 12499541.
- Rudisser, S.; Tinoco, I. Jr. (2000). "Solution structure of Cobalt(III)hexammine complexed to the GAAA tetraloop, and metal-ion binding to G.A mismatches". J. Mol. Biol. 295 (5): 1211–1232. doi:10.1006/jmbi.1999.3421. PMID 10653698.
- McPherson, Alexander (2002). Introduction to Macromolecular Crystallography. John Wiley & Sons. ISBN 0-471-25122-4.
- Brennant, R. G.; Westhof, E.; Sundaralingam, M. (1986). "Structure of a Z-DNA with Two Different Backbone Chain Conformations. Stabilization of the Decadeoxyoligonucleotide d(CGTACGTACG) by [CO(NH3)6]Binding to the Guanine". Journal of Biomolecular Structure and Dynamics. 3 (4): 649–665. doi:10.1080/07391102.1986.10508453. PMID 3271042.