| Placenta | |
|---|---|
 Human placenta from just after birth with the umbilical cord in place Human placenta from just after birth with the umbilical cord in place | |
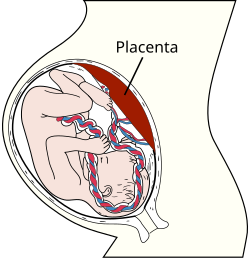 Human placenta shown in uterus connected to fetus with umbilical cord Human placenta shown in uterus connected to fetus with umbilical cord | |
| Details | |
| Precursor | Decidua basalis, chorion frondosum |
| Identifiers | |
| Latin | placenta |
| MeSH | D010920 |
| TE | E5.11.3.1.1.0.5 |
| Anatomical terminology[edit on Wikidata] | |
The placenta (pl.: placentas or placentae) is a temporary embryonic and later fetal organ that begins developing from the blastocyst shortly after implantation. It plays critical roles in facilitating nutrient, gas and waste exchange between the physically separate maternal and fetal circulations, and is an important endocrine organ, producing hormones that regulate both maternal and fetal physiology during pregnancy. The placenta connects to the fetus via the umbilical cord, and on the opposite aspect to the maternal uterus in a species-dependent manner. In humans, a thin layer of maternal decidual (endometrial) tissue comes away with the placenta when it is expelled from the uterus following birth (sometimes incorrectly referred to as the 'maternal part' of the placenta). Placentas are a defining characteristic of placental mammals, but are also found in marsupials and some non-mammals with varying levels of development.
Mammalian placentas probably first evolved about 150 million to 200 million years ago. The protein syncytin, found in the outer barrier of the placenta (the syncytiotrophoblast) between mother and fetus, has a certain RNA signature in its genome that has led to the hypothesis that it originated from an ancient retrovirus: essentially a virus that helped pave the transition from egg-laying to live-birth.
The word placenta comes from the Latin word for a type of cake, from Greek πλακόεντα/πλακοῦντα plakóenta/plakoúnta, accusative of πλακόεις/πλακούς plakóeis/plakoús, "flat, slab-like", with reference to its round, flat appearance in humans. The classical plural is placentae, but the form placentas is more common in modern English.
Evolution and phylogenetic diversity
The placenta has evolved independently multiple times, probably starting in fish, where it originated multiple times, including the genus Poeciliopsis. Placentation has also evolved in some reptiles.
The mammalian placenta evolved more than 100 million years ago and was a critical factor in the explosive diversification of placental mammals. Although all mammalian placentas have the same functions, there are important differences in structure and function in different groups of mammals. For example, human, bovine, equine and canine placentas are very different at both the gross and the microscopic levels. Placentas of these species also differ in their ability to provide maternal immunoglobulins to the fetus.
Structure
Placental mammals, including humans, have a chorioallantoic placenta that forms from the chorion and allantois. In humans, the placenta averages 22 cm (9 inch) in length and 2–2.5 cm (0.8–1 inch) in thickness, with the center being the thickest, and the edges being the thinnest. It typically weighs approximately 500 grams (just over 1 lb). It has a dark reddish-blue or crimson color. It connects to the fetus by an umbilical cord of approximately 55–60 cm (22–24 inch) in length, which contains two umbilical arteries and one umbilical vein. The umbilical cord inserts into the chorionic plate (has an eccentric attachment). Vessels branch out over the surface of the placenta and further divide to form a network covered by a thin layer of cells. This results in the formation of villous tree structures. On the maternal side, these villous tree structures are grouped into lobules called cotyledons. In humans, the placenta usually has a disc shape, but size varies vastly between different mammalian species.
The placenta occasionally takes a form in which it comprises several distinct parts connected by blood vessels. The parts, called lobes, may number two, three, four, or more. Such placentas are described as bilobed/bilobular/bipartite, trilobed/trilobular/tripartite, and so on. If there is a clearly discernible main lobe and auxiliary lobe, the latter is called a succenturiate placenta. Sometimes the blood vessels connecting the lobes get in the way of fetal presentation during labor, which is called vasa previa.
Gene and protein expression
Further information: Bioinformatics § Gene and protein expressionAbout 20,000 protein coding genes are expressed in human cells and 70% of these genes are expressed in the normal mature placenta. Some 350 of these genes are more specifically expressed in the placenta and fewer than 100 genes are highly placenta specific. The corresponding specific proteins are mainly expressed in trophoblasts and have functions related to pregnancy. Examples of proteins with elevated expression in placenta compared to other organs and tissues are PEG10 and the cancer testis antigen PAGE4 and expressed in cytotrophoblasts, CSH1 and KISS1 expressed in syncytiotrophoblasts, and PAPPA2 and PRG2 expressed in extravillous trophoblasts.
Physiology
Development


The placenta begins to develop upon implantation of the blastocyst into the maternal endometrium, very early on in pregnancy at about week 4.
The outer layer of the late blastocyst, is formed of trophoblasts, cells that form the outer layer of the placenta. This outer layer is divided into two further layers: the underlying cytotrophoblast layer and the overlying syncytiotrophoblast layer. The syncytiotrophoblast is a multinucleated continuous cell layer that covers the surface of the placenta. It forms as a result of differentiation and fusion of the underlying cytotrophoblasts, a process that continues throughout placental development. The syncytiotrophoblast contributes to the barrier function of the placenta.
The placenta grows throughout pregnancy. Development of the maternal blood supply to the placenta is complete by the end of the first trimester of pregnancy week 14 (DM).
Placental circulation
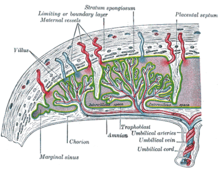
Maternal placental circulation
In preparation for implantation of the blastocyst, the endometrium undergoes decidualization. Spiral arteries in the decidua are remodeled so that they become less convoluted and their diameter is increased. The increased diameter and straighter flow path both act to increase maternal blood flow to the placenta. There is relatively high pressure as the maternal blood fills intervillous space through these spiral arteries which bathe the fetal villi in blood, allowing an exchange of gases to take place. In humans and other hemochorial placentals, the maternal blood comes into direct contact with the fetal chorion, though no fluid is exchanged. As the pressure decreases between pulses, the deoxygenated blood flows back through the endometrial veins.
Maternal blood flow begins between days 5–12, and is approximately 600–700 ml/min at term.
Fetoplacental circulation
Further information: Fetal circulationDeoxygenated fetal blood passes through umbilical arteries to the placenta. At the junction of umbilical cord and placenta, the umbilical arteries branch radially to form chorionic arteries. Chorionic arteries, in turn, branch into cotyledon arteries. In the villi, these vessels eventually branch to form an extensive arterio-capillary-venous system, bringing the fetal blood extremely close to the maternal blood; but no intermingling of fetal and maternal blood occurs ("placental barrier").
Endothelin and prostanoids cause vasoconstriction in placental arteries, while nitric oxide causes vasodilation. On the other hand, there is no neural vascular regulation, and catecholamines have only little effect.
The fetoplacental circulation is vulnerable to persistent hypoxia or intermittent hypoxia and reoxygenation, which can lead to generation of excessive free radicals. This may contribute to pre-eclampsia and other pregnancy complications. It is proposed that melatonin plays a role as an antioxidant in the placenta.
This begins at day 17–22.
Birth
Main article: Placental expulsionPlacental expulsion begins as a physiological separation from the wall of the uterus. The period from just after the child is born until just after the placenta is expelled is called the "third stage of labor".
Placental expulsion can be managed actively, for example by giving oxytocin via intramuscular injection followed by cord traction to assist in delivering the placenta. Alternatively, it can be managed expectantly, allowing the placenta to be expelled without medical assistance. Blood loss and the risk of postpartum bleeding may be reduced in women offered active management of the third stage of labour, however there may be adverse effects and more research is necessary.
The habit is to cut the cord immediately after birth, but it may be no medical reason to do this; on the contrary, not cutting the cord could sometimes help the baby in its adaptation to extrauterine life, for preterm infants.
Microbiome
Main article: Placental microbiomeThe placenta is traditionally thought to be sterile, but recent research suggests that a resident, non-pathogenic, and diverse population of microorganisms may be present in healthy tissue. However, whether these microbes exist or are clinically important is highly controversial and is the subject of active research.
Physiology of placenta
Nutrition and gas exchange
The placenta intermediates the transfer of nutrients between mother and fetus. The perfusion of the intervillous spaces of the placenta with maternal blood allows the transfer of nutrients and oxygen from the mother to the fetus and the transfer of waste products and carbon dioxide back from the fetus to the maternal blood. Nutrient transfer to the fetus can occur via both active and passive transport. Placental nutrient metabolism was found to play a key role in limiting the transfer of some nutrients. Adverse pregnancy situations, such as those involving maternal diabetes or obesity, can increase or decrease levels of nutrient transporters in the placenta potentially resulting in overgrowth or restricted growth of the fetus.
Excretion
Waste products excreted from the fetus such as urea, uric acid, and creatinine are transferred to the maternal blood by diffusion across the placenta.
Immunity
The placenta functions as a selective barrier between maternal and fetal cells, preventing maternal blood, proteins and microbes (including bacteria and most viruses) from crossing the maternal-fetal barrier. Deterioration in placental functioning, referred to as placental insufficiency, may be related to mother-to-child transmission of some infectious diseases. A very small number of viruses including rubella virus, Zika virus and cytomegalovirus (CMV) can travel across the placental barrier, generally taking advantage of conditions at certain gestational periods as the placenta develops. CMV and Zika travel from the maternal bloodstream via placental cells to the fetal bloodstream.
Beginning as early as 13 weeks of gestation, and increasing linearly, with the largest transfer occurring in the third trimester, IgG antibodies can pass through the human placenta, providing protection to the fetus in utero. This passive immunity lingers for several months after birth, providing the newborn with a carbon copy of the mother's long-term humoral immunity to see the infant through the crucial first months of extrauterine life. IgM antibodies, because of their larger size, cannot cross the placenta, one reason why infections acquired during pregnancy can be particularly hazardous for the fetus.
Hormonal regulation
- The first hormone released by the placenta is called the human chorionic gonadotropin (hCG) hormone. This is responsible for stopping the process at the end of menses when the corpus luteum ceases activity and atrophies. If hCG did not interrupt this process, it would lead to spontaneous abortion of the fetus. The corpus luteum also produces and releases progesterone and estrogen, and hCG stimulates it to increase the amount that it releases. hCG is the indicator of pregnancy that pregnancy tests look for. These tests will work when menses has not occurred or after implantation has happened on days seven to ten. hCG may also have an anti-antibody effect, protecting it from being rejected by the mother's body. hCG also assists the male fetus by stimulating the testes to produce testosterone, which is the hormone needed to allow the sex organs of the male to grow.
- Progesterone helps the embryo implant by assisting passage through the fallopian tubes. It also affects the fallopian tubes and the uterus by stimulating an increase in secretions necessary for fetal nutrition. Progesterone, like hCG, is necessary to prevent spontaneous abortion because it prevents contractions of the uterus and is necessary for implantation.
- Estrogen is a crucial hormone in the process of proliferation. This involves the enlargement of the breasts and uterus, allowing for growth of the fetus and production of milk. Estrogen is also responsible for increased blood supply towards the end of pregnancy through vasodilation. The levels of estrogen during pregnancy can increase so that they are thirty times what a non-pregnant woman mid-cycles estrogen level would be.
- Human placental lactogen (hPL) is a hormone used in pregnancy to develop fetal metabolism and general growth and development. Human placental lactogen works with growth hormone to stimulate insulin-like growth factor production and regulating intermediary metabolism. In the fetus, hPL acts on lactogenic receptors to modulate embryonic development, metabolism and stimulate production of IGF, insulin, surfactant and adrenocortical hormones. hPL values increase with multiple pregnancies, intact molar pregnancy, diabetes and Rh incompatibility. They are decreased with toxemia, choriocarcinoma, and Placental insufficiency.
Immunological barrier
Further information: Immune tolerance in pregnancyThe placenta and fetus may be regarded as a foreign body inside the mother and must be protected from the normal immune response of the mother that would cause it to be rejected. The placenta and fetus are thus treated as sites of immune privilege, with immune tolerance.
For this purpose, the placenta uses several mechanisms :
- It secretes neurokinin B-containing phosphocholine molecules. This is the same mechanism used by parasitic nematodes to avoid detection by the immune system of their host.
- There is presence of small lymphocytic suppressor cells in the fetus that inhibit maternal cytotoxic T cells by inhibiting the response to interleukin 2.
However, the placental barrier is not the sole means of evading the immune system, as foreign fetal cells also persist in the maternal circulation, on the other side of the placental barrier.
DNA methylation
The trophoblast is the outer layer of cells of the blastocyst (see day 9 in Figure, above, showing the initial stages of human embryogenesis). Placental trophoblast cells have a unique genome-wide DNA methylation pattern determined by de novo methyltransferases during embryogenesis. This methylation pattern is principally required to regulate placental development and function, which in turn is critical for embryo survival.
Other
The placenta also provides a reservoir of blood for the fetus, delivering blood to it in case of hypotension and vice versa, comparable to a capacitor.

Clinical significance
Main article: Placental disease
Numerous pathologies can affect the placenta.
- Placenta accreta, when the placenta implants too deeply, all the way to the actual muscle of uterine wall (without penetrating it)
- Placenta praevia, when the placement of the placenta is too close to or blocks the cervix
- Placental abruption, premature detachment of the placenta
- Placentitis, inflammation of the placenta, such as by TORCH infections.
Society and culture
The placenta often plays an important role in various cultures, with many societies conducting rituals regarding its disposal. In the Western world, the placenta is most often incinerated.
Some cultures bury the placenta for various reasons. The Māori of New Zealand traditionally bury the placenta from a newborn child to emphasize the relationship between humans and the earth. Likewise, the Navajo bury the placenta and umbilical cord at a specially chosen site, particularly if the baby dies during birth. In Cambodia and Costa Rica, burial of the placenta is believed to protect and ensure the health of the baby and the mother. If a mother dies in childbirth, the Aymara of Bolivia bury the placenta in a secret place so that the mother's spirit will not return to claim her baby's life.
The placenta is believed by some communities to have power over the lives of the baby or its parents. The Kwakiutl of British Columbia bury girls' placentas to give the girl skill in digging clams, and expose boys' placentas to ravens to encourage future prophetic visions. In Turkey, the proper disposal of the placenta and umbilical cord is believed to promote devoutness in the child later in life. In Transylvania and Japan, interaction with a disposed placenta is thought to influence the parents' future fertility.
Several cultures believe the placenta to be or have been alive, often a relative of the baby. Nepalese think of the placenta as a friend of the baby; the orang Asli and Malay populations in Malay Peninsula regard it as the baby's older sibling. Native Hawaiians believe that the placenta is a part of the baby, and traditionally plant it with a tree that can then grow alongside the child. Various cultures in Indonesia, such as Javanese and Malay, believe that the placenta has a spirit and needs to be buried outside the family house. Some Malays would bury the baby's placenta with a pencil (if it is a boy) or a needle and thread (if it is a girl).
In some cultures, the placenta is eaten, a practice known as placentophagy. In some eastern cultures, such as China, the dried placenta (ziheche 紫河车, literally "purple river car") is thought to be a healthful restorative and is sometimes used in preparations of traditional Chinese medicine and various health products. The practice of human placentophagy has become a more recent trend in western cultures and is not without controversy; its practice being considered cannibalism is debated.
Some cultures have alternative uses for placenta that include the manufacturing of cosmetics, pharmaceuticals and food.
Additional images
-
 Human placenta immediately post birth.
Human placenta immediately post birth.
-
 Fetus of about 8 weeks, enclosed in the amnion. Magnified a little over two diameters.
Fetus of about 8 weeks, enclosed in the amnion. Magnified a little over two diameters.
-
 Placenta with attached fetal membranes, ruptured at the margin at the left in the image
Placenta with attached fetal membranes, ruptured at the margin at the left in the image
-
 Micrograph of a placental infection (CMV placentitis)
Micrograph of a placental infection (CMV placentitis)
-
 Micrograph of CMV placentitis
Micrograph of CMV placentitis
-
 A 3D Power Doppler image of vasculature in 20-week placenta
A 3D Power Doppler image of vasculature in 20-week placenta
-
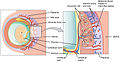 Schematic view of the placenta
Schematic view of the placenta
-
Maternal side of a whole human placenta, just after birth
-
Fetal side of same placenta
-
 Close-up of umbilical attachment to fetal side of freshly delivered placenta
Close-up of umbilical attachment to fetal side of freshly delivered placenta
-
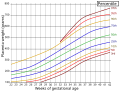 Placenta weight by gestational age
Placenta weight by gestational age
-
 Ziheche (紫河车), dried human placenta used in traditional Chinese medicine
Ziheche (紫河车), dried human placenta used in traditional Chinese medicine
See also
References
- Jin M, Xu Q, Li J, Xu S, Tang C (September 2022). "Micro-RNAs in Human Placenta: Tiny Molecules, Immense Power". Molecules. 27 (18): 5943. doi:10.3390/molecules27185943. PMC 9501247. PMID 36144676.
- Pough FH, Andrews RM, Cadle JE, Crump ML, Savitsky AH, Wells KD (2004). Herpetology (3rd ed.). Pearson. ISBN 978-0-13-100849-6.
- Mitra A (31 January 2020). "How the placenta evolved from an ancient virus". WHYY. Retrieved 9 March 2020.
- Chuong EB (October 2018). "The placenta goes viral: Retroviruses control gene expression in pregnancy". PLOS Biology. 16 (10): e3000028. doi:10.1371/journal.pbio.3000028. PMC 6177113. PMID 30300353.
- Villarreal LP (January 2016). "Viruses and the placenta: the essential virus first view". APMIS. 124 (1–2): 20–30. doi:10.1111/apm.12485. PMID 26818259. S2CID 12042851.
- Henry George Liddell, Robert Scott, "A Greek-English Lexicon", at Perseus Archived 2012-04-05 at the Wayback Machine.
- "placenta" Archived 2016-01-30 at the Wayback Machine. Online Etymology Dictionary.
- Reznick DN, Mateos M, Springer MS (November 2002). "Independent origins and rapid evolution of the placenta in the fish genus Poeciliopsis". Science. 298 (5595): 1018–1020. Bibcode:2002Sci...298.1018R. doi:10.1126/science.1076018. PMID 12411703. S2CID 2372572.
- Blackburn DG, Avanzati AM, Paulesu L (November 2015). "Classics revisited. History of reptile placentology: Studiati's early account of placentation in a viviparous lizard". Placenta. 36 (11): 1207–1211. doi:10.1016/j.placenta.2015.09.013. PMID 26474917.
- Ayala FJ (May 2007). "Darwin's greatest discovery: design without designer". Proceedings of the National Academy of Sciences of the United States of America. 104 (Suppl 1): 8567–8573. doi:10.1073/pnas.0701072104. PMC 1876431. PMID 17494753.
- Bowen R. "Implantation and Development of the Placenta: Introduction and Index". Pathophysiology of the Reproductive System. Colorado State. Retrieved 7 July 2019.
- Yetter JF (March 1998). "Examination of the placenta". American Family Physician. 57 (5): 1045–54. PMID 9518951. Archived from the original on 2011-10-16.
- "Placental Structure and Classification". Colorado State. Archived from the original on 2016-02-11.
- Fujikura T, Benson RC, Driscoll SG (August 1970). "The bipartite placenta and its clinical features". American Journal of Obstetrics and Gynecology. 107 (7): 1013–1017. doi:10.1016/0002-9378(70)90621-6. PMID 5429965.
Bipartite placenta represented 4.2 per cent (366 of 8,505) of placentas of white women at the Boston Hospital for Women who were enrolled in the Collaborative Project.
- "The human proteome in placenta - The Human Protein Atlas". www.proteinatlas.org. Archived from the original on 2017-09-26. Retrieved 2017-09-26.
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. (January 2015). "Proteomics. Tissue-based map of the human proteome". Science. 347 (6220): 1260419. doi:10.1126/science.1260419. PMID 25613900. S2CID 802377.
- ^ "Stages of Development of the Fetus - Women's Health Issues". Merck Manuals Consumer Version. Retrieved 2022-06-12.
- "How Your Fetus Grows During Pregnancy". www.acog.org. Retrieved 2022-06-12.
- Dashe JS, Bloom SL, Spong CY, Hoffman BL (2018). Williams Obstetrics. McGraw Hill Professional. ISBN 978-1-259-64433-7.
- "Placental blood circulation". Online course in embryology for medical students. Universities of Fribourg, Lausanne and Bern (Switzerland). Archived from the original on 2011-09-28.
- ^ Kiserud T, Acharya G (December 2004). "The fetal circulation". Prenatal Diagnosis. 24 (13): 1049–1059. doi:10.1002/pd.1062. PMID 15614842. S2CID 25040285.
- ^ Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA (2013). "Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology". Human Reproduction Update. 20 (2): 293–307. doi:10.1093/humupd/dmt054. PMID 24132226.
- Williams book of obsteritcis.
- Begley CM, Gyte GM, Devane D, McGuire W, Weeks A, Biesty LM (February 2019). "Active versus expectant management for women in the third stage of labour". The Cochrane Database of Systematic Reviews. 2019 (2): CD007412. doi:10.1002/14651858.CD007412.pub5. PMC 6372362. PMID 30754073.
- Mercer JS, Vohr BR, Erickson-Owens DA, Padbury JF, Oh W (January 2010). "Seven-month developmental outcomes of very low birth weight infants enrolled in a randomized controlled trial of delayed versus immediate cord clamping". Journal of Perinatology. 30 (1): 11–16. doi:10.1038/jp.2009.170. PMC 2799542. PMID 19847185.
- Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J (April 2017). "A critical assessment of the "sterile womb" and "in utero colonization" hypotheses: implications for research on the pioneer infant microbiome". Microbiome. 5 (1): 48. doi:10.1186/s40168-017-0268-4. PMC 5410102. PMID 28454555.
- Mor G, Kwon JY (October 2015). "Trophoblast-microbiome interaction: a new paradigm on immune regulation". American Journal of Obstetrics and Gynecology. 213 (4 Suppl): S131–S137. doi:10.1016/j.ajog.2015.06.039. PMC 6800181. PMID 26428492.
- Prince AL, Antony KM, Chu DM, Aagaard KM (October 2014). "The microbiome, parturition, and timing of birth: more questions than answers". Journal of Reproductive Immunology. 104–105: 12–19. doi:10.1016/j.jri.2014.03.006. PMC 4157949. PMID 24793619.
- Hornef M, Penders J (May 2017). "Does a prenatal bacterial microbiota exist?". Mucosal Immunology. 10 (3): 598–601. doi:10.1038/mi.2016.141. PMID 28120852.
- Wright C, Sibley CP (2011). "Placental Transfer in Health and Disease". In Kay H, Nelson M, Yuping W (eds.). The Placenta: From Development to Disease. John Wiley and Sons. pp. 66. ISBN 978-1-4443-3366-4.
- Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG (February 2017). "The influence of placental metabolism on fatty acid transfer to the fetus". Journal of Lipid Research. 58 (2): 443–454. doi:10.1194/jlr.P072355. PMC 5282960. PMID 27913585.
- Kappen C, Kruger C, MacGowan J, Salbaum JM (2012). "Maternal diet modulates placenta growth and gene expression in a mouse model of diabetic pregnancy". PLOS ONE. 7 (6): e38445. Bibcode:2012PLoSO...738445K. doi:10.1371/journal.pone.0038445. PMC 3372526. PMID 22701643.
- ^ Madhusoodanan J (October 10, 2018). "Break on through: How some viruses infect the placenta". Knowable Magazine. doi:10.1146/knowable-101018-1.
- Erlebacher A (2013-03-21). "Immunology of the maternal-fetal interface". Annual Review of Immunology. 31 (1): 387–411. doi:10.1146/annurev-immunol-032712-100003. PMID 23298207.
- Pereira L (September 2018). "Congenital Viral Infection: Traversing the Uterine-Placental Interface". Annual Review of Virology. 5 (1): 273–299. doi:10.1146/annurev-virology-092917-043236. PMID 30048217. S2CID 51724379.
- Arora N, Sadovsky Y, Dermody TS, Coyne CB (May 2017). "Microbial Vertical Transmission during Human Pregnancy". Cell Host & Microbe. 21 (5): 561–567. doi:10.1016/j.chom.2017.04.007. PMC 6148370. PMID 28494237.
- Robbins JR, Bakardjiev AI (February 2012). "Pathogens and the placental fortress". Current Opinion in Microbiology. 15 (1): 36–43. doi:10.1016/j.mib.2011.11.006. PMC 3265690. PMID 22169833.
- Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M (2012). "IgG placental transfer in healthy and pathological pregnancies". Clinical & Developmental Immunology. 2012: 985646. doi:10.1155/2012/985646. PMC 3251916. PMID 22235228.
- Simister NE, Story CM (December 1997). "Human placental Fc receptors and the transmission of antibodies from mother to fetus". Journal of Reproductive Immunology. 37 (1): 1–23. doi:10.1016/s0165-0378(97)00068-5. PMID 9501287.
- Pillitteri A (2009). Maternal and Child Health Nursing: Care of the Childbearing and Childrearing Family. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 202. ISBN 978-1-58255-999-5.
- "What infections can affect pregnancy?". National Institute of Child Health and Human Development. 27 April 2021. Retrieved 25 June 2021.
- Handwerger S, Freemark M (April 2000). "The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development". Journal of Pediatric Endocrinology & Metabolism. 13 (4): 343–356. doi:10.1515/jpem.2000.13.4.343. PMID 10776988. S2CID 28778529.
- "Human Placental Lactogen". www.ucsfhealth.org. May 17, 2009. Archived from the original on April 29, 2017. Retrieved July 21, 2017.
- "Placenta 'fools body's defences'". BBC News. 10 November 2007. Archived from the original on 29 April 2012.
- Clark DA, Chaput A, Tutton D (March 1986). "Active suppression of host-vs-graft reaction in pregnant mice. VII. Spontaneous abortion of allogeneic CBA/J x DBA/2 fetuses in the uterus of CBA/J mice correlates with deficient non-T suppressor cell activity". Journal of Immunology. 136 (5): 1668–1675. doi:10.4049/jimmunol.136.5.1668. PMID 2936806. S2CID 22815679.
- Williams Z, Zepf D, Longtine J, Anchan R, Broadman B, Missmer SA, Hornstein MD (June 2009). "Foreign fetal cells persist in the maternal circulation". Fertility and Sterility. 91 (6): 2593–2595. doi:10.1016/j.fertnstert.2008.02.008. PMID 18384774.
- ^ Andrews S, Krueger C, Mellado-Lopez M, Hemberger M, Dean W, Perez-Garcia V, Hanna CW (January 2023). "Mechanisms and function of de novo DNA methylation in placental development reveals an essential role for DNMT3B". Nat Commun. 14 (1): 371. doi:10.1038/s41467-023-36019-9. PMC 9870994. PMID 36690623.
- Assad RS, Lee FY, Hanley FL (May 2001). "Placental compliance during fetal extracorporeal circulation". Journal of Applied Physiology. 90 (5): 1882–1886. doi:10.1152/jappl.2001.90.5.1882. PMID 11299282. S2CID 8785947.
- ^ "Why eat a placenta?". BBC News. 18 April 2006.
- Metge J (2005). "Working In/Playing With Three Languages". Sites: A Journal of Social Anthropology and Cultural Studies. 2 (2): 83–90. doi:10.11157/sites-vol2iss2id65.
- Francisco E (3 December 2004). "Bridging the Cultural Divide in Medicine". Science.
- Shepardson M (1978). "Changes in Navajo mortuary practices and beliefs". American Indian Quarterly. 4 (4): 383–396. doi:10.2307/1184564. JSTOR 1184564. PMID 11614175.
- ^ Buckley SJ. "Placenta Rituals and Folklore from around the World". Mothering. Archived from the original on 6 January 2008. Retrieved 7 January 2008.
- Davenport A (June 2005). "The Love Offer". Johns Hopkins Magazine. 57 (3).
- ^ Barakbah A (2017). Ensiklopedia Perbidanan Melayu. Universiti Islam Malaysia Press. pp. 236–237. ISBN 978-967-13305-9-3.
- Falcao R. "Medicinal Uses of the Placenta". Archived from the original on 5 December 2008. Retrieved 25 November 2008.
- Kroløkke C, Dickinson E, Foss KA (May 2018). "The placenta economy: From trashed to treasured bio-products". European Journal of Women's Studies. 25 (2): 138–153. doi:10.1177/1350506816679004. ISSN 1350-5068. S2CID 151874106.
- "Placental Weights: Means, Standard Deviations, and Percentiles by Gestational Age". Placental and Gestational Pathology. 2017. p. 336. doi:10.1017/9781316848616.039. ISBN 978-1-316-84861-6.
External links
- The placenta-specific proteome at the Human Protein Atlas
- The Placenta, gynob.com, with quotes from Williams Obstetrics, 18th Edition, F. Gary Cunningham, M.D., Paul C. MacDonald, M.D., Norman F. Grant, M.D., Appleton & Lange, Publishers.
| Membranes of the fetus and embryo | |
|---|---|
| Embryo | |
| Fetus | |
| Circulatory | |
| Other | |
| Development of the circulatory system | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Heart |
| ||||||||
| Vessels |
| ||||||||
| Extraembryonic hemangiogenesis | |||||||||
| Fetal circulation | |||||||||