"ADD", "ADHD", and "Hyperactive" redirect here. For other uses, see ADD (disambiguation), ADHD (disambiguation), and Hyperactive (disambiguation).
Medical condition
| Attention deficit hyperactivity disorder | |
|---|---|
| Other names | Formerly: Attention deficit disorder (ADD), hyperkinetic disorder (HD) |
 | |
| ADHD arises from maldevelopment in brain regions such as the prefrontal cortex, basal ganglia and anterior cingulate cortex, which regulate the executive functions necessary for human self-regulation. | |
| Specialty | |
| Symptoms | |
| Usual onset | In most cases at least some ADHD symptoms and impairments onset prior to age 12. |
| Causes | Genetic (inherited, de novo) and to a lesser extent, environmental factors (exposure to biohazards during pregnancy, traumatic brain injury) |
| Diagnostic method | Based on impairing symptoms after other possible causes have been ruled out |
| Differential diagnosis | |
| Treatment |
|
| Medication | |
| Frequency | 0.8–1.5% (2019, using DSM-IV-TR and ICD-10) |
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by executive dysfunction occasioning symptoms of inattention, hyperactivity, impulsivity and emotional dysregulation that are excessive and pervasive, impairing in multiple contexts, and developmentally-inappropriate.
ADHD symptoms arise from executive dysfunction, and emotional dysregulation is often considered a core symptom. Impairments resulting from deficits in self-regulation such as time management, inhibition, and sustained attention can include poor professional performance, relationship difficulties, and numerous health risks, collectively predisposing to a diminished quality of life and a direct average reduction in life expectancy of 13 years. The disorder costs society hundreds of billions of US dollars each year, worldwide. It is associated with other neurodevelopmental and mental disorders as well as non-psychiatric disorders, which can cause additional impairment.
While people with ADHD often struggle to initiate work and persist on tasks with delayed consequences, this may not be evident in contexts they find intrinsically interesting and immediately rewarding, potentiating hyperfocus (a more colloquial term) or perseverative responding. This mental state is often hard to disengage from and is related to risks such as for internet addiction and types of offending behaviour.
ADHD represents the extreme lower end of the continuous dimensional trait (bell curve) of executive functioning and self-regulation, which is supported by twin, brain imaging and molecular genetic studies.
The precise causes of ADHD are unknown in most individual cases. Meta-analyses have shown that the disorder is primarily genetic with a heritability rate of 70-80%, where risk factors are highly accumulative. The environmental risks are not related to social or familial factors; they exert their effects very early in life, in the prenatal or early postnatal period. However, in rare cases, ADHD can be caused by a single event including traumatic brain injury, exposure to biohazards during pregnancy, or a major genetic mutation. There is no biologically distinct adult-onset ADHD except for when ADHD occurs after traumatic brain injury.
Signs and symptoms
Inattention, hyperactivity (restlessness in adults), disruptive behaviour, and impulsivity are common in ADHD. Academic difficulties are frequent, as are problems with relationships. The signs and symptoms can be difficult to define, as it is hard to draw a line at where normal levels of inattention, hyperactivity, and impulsivity end and significant levels requiring interventions begin.
According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and its text revision (DSM-5-TR), symptoms must be present for six months or more to a degree that is much greater than others of the same age. This requires at least six symptoms of either inattention or hyperactivity/impulsivity for those under 17 and at least five symptoms for those 17 years or older. The symptoms must be present in at least two settings (e.g., social, school, work, or home), and must directly interfere with or reduce quality of functioning. Additionally, several symptoms must have been present before age 12. The DSM-5 's required age of onset of symptoms is 12 years. However, research indicates the age of onset should not be interpreted as a prerequisite for diagnosis given contextual exceptions.
Presentations
ADHD is divided into three primary presentations:
- predominantly inattentive (ADHD-PI or ADHD-I)
- predominantly hyperactive-impulsive (ADHD-PH or ADHD-HI)
- combined presentation (ADHD-C).
The table "Symptoms" lists the symptoms for ADHD-I and ADHD-HI from two major classification systems. Symptoms which can be better explained by another psychiatric or medical condition which an individual has are not considered to be a symptom of ADHD for that person. In DSM-5, subtypes were discarded and reclassified as presentations of the disorder that change over time.
| Presentations | DSM-5 and DSM-5-TR symptoms | ICD-11 symptoms |
|---|---|---|
| Inattention | Six or more of the following symptoms in children, and five or more in adults, excluding situations where these symptoms are better explained by another psychiatric or medical condition:
|
Multiple symptoms of inattention that directly negatively impact occupational, academic or social functioning. Symptoms may not be present when engaged in highly stimulating tasks with frequent rewards. Symptoms are generally from the following clusters:
The individual may also meet the criteria for hyperactivity-impulsivity, but the inattentive symptoms are predominant. |
| Hyperactivity-Impulsivity | Six or more of the following symptoms in children, and five or more in adults, excluding situations where these symptoms are better explained by another psychiatric or medical condition:
|
Multiple symptoms of hyperactivity/impulsivity that directly negatively impact occupational, academic or social functioning. Typically, these tend to be most apparent in environments with structure or which require self-control. Symptoms are generally from the following clusters:
The individual may also meet the criteria for inattention, but the hyperactive-impulsive symptoms are predominant. |
| Combined | Meet the criteria for both inattentive and hyperactive-impulsive ADHD. | Criteria are met for both inattentive and hyperactive-impulsive ADHD, with neither clearly predominating. |
Girls and women with ADHD tend to display fewer hyperactivity and impulsivity symptoms but more symptoms of inattention and distractibility.
Symptoms are expressed differently and more subtly as the individual ages. Hyperactivity tends to become less overt with age and turns into inner restlessness, difficulty relaxing or remaining still, talkativeness or constant mental activity in teens and adults with ADHD. Impulsivity in adulthood may appear as thoughtless behaviour, impatience, irresponsible spending and sensation-seeking behaviours, while inattention may appear as becoming easily bored, difficulty with organization, remaining on task and making decisions, and sensitivity to stress.
Although not listed as an official symptom, emotional dysregulation or mood lability is generally understood to be a common symptom of ADHD. People with ADHD of all ages are more likely to have problems with social skills, such as social interaction and forming and maintaining friendships. This is true for all presentations. About half of children and adolescents with ADHD experience social rejection by their peers compared to 10–15% of non-ADHD children and adolescents. People with attention deficits are prone to having difficulty processing verbal and nonverbal language which can negatively affect social interaction. They may also drift off during conversations, miss social cues, and have trouble learning social skills.
Difficulties managing anger are more common in children with ADHD, as are delays in speech, language and motor development. Poorer handwriting is more common in children with ADHD. Poor handwriting can be a symptom of ADHD in itself due to decreased attentiveness. When this is a pervasive problem, it may also be attributable to dyslexia or dysgraphia. There is significant overlap in the symptomatologies of ADHD, dyslexia, and dysgraphia, and 3 in 10 people diagnosed with dyslexia experience co-occurring ADHD. Although it causes significant difficulty, many children with ADHD have an attention span equal to or greater than that of other children for tasks and subjects they find interesting.
IQ test performance
Certain studies have found that people with ADHD tend to have lower scores on intelligence quotient (IQ) tests. The significance of this is controversial due to the differences between people with ADHD and the difficulty determining the influence of symptoms, such as distractibility, on lower scores rather than intellectual capacity. In studies of ADHD, higher IQs may be over-represented because many studies exclude individuals who have lower IQs despite those with ADHD scoring on average nine points lower on standardised intelligence measures. However, other studies contradict this, saying that in individuals with high intelligence, there is an increased risk of a missed ADHD diagnosis, possibly because of compensatory strategies in said individuals.
Studies of adults suggest that negative differences in intelligence are not meaningful and may be explained by associated health problems.
Comorbidities
Psychiatric comorbidities
In children, ADHD occurs with other disorders about two-thirds of the time.
Other neurodevelopmental conditions are common comorbidities. Autism spectrum disorder (ASD), co-occurring at a rate of 21% in those with ADHD, affects social skills, ability to communicate, behaviour, and interests. Learning disabilities have been found to occur in about 20–30% of children with ADHD. Learning disabilities can include developmental speech and language disorders, and academic skills disorders. ADHD, however, is not considered a learning disability, but it very frequently causes academic difficulties. Intellectual disabilities and Tourette's syndrome are also common.
ADHD is often comorbid with disruptive, impulse control, and conduct disorders. Oppositional defiant disorder (ODD) occurs in about 25% of children with an inattentive presentation and 50% of those with a combined presentation. It is characterised by angry or irritable mood, argumentative or defiant behaviour and vindictiveness which are age-inappropriate. Conduct disorder (CD) occurs in about 25% of adolescents with ADHD. It is characterised by aggression, destruction of property, deceitfulness, theft and violations of rules. Adolescents with ADHD who also have CD are more likely to develop antisocial personality disorder in adulthood. Brain imaging supports that CD and ADHD are separate conditions: conduct disorder was shown to reduce the size of one's temporal lobe and limbic system, and increase the size of one's orbitofrontal cortex, whereas ADHD was shown to reduce connections in the cerebellum and prefrontal cortex more broadly. Conduct disorder involves more impairment in motivation control than ADHD. Intermittent explosive disorder is characterised by sudden and disproportionate outbursts of anger and co-occurs in individuals with ADHD more frequently than in the general population.
Anxiety and mood disorders are frequent comorbidities. Anxiety disorders have been found to occur more commonly in the ADHD population, as have mood disorders (especially bipolar disorder and major depressive disorder). Boys diagnosed with the combined ADHD subtype are more likely to have a mood disorder. Adults and children with ADHD sometimes also have bipolar disorder, which requires careful assessment to accurately diagnose and treat both conditions.
Sleep disorders and ADHD commonly co-exist. They can also occur as a side effect of medications used to treat ADHD. In children with ADHD, insomnia is the most common sleep disorder with behavioural therapy being the preferred treatment. Problems with sleep initiation are common among individuals with ADHD but often they will be deep sleepers and have significant difficulty getting up in the morning. Melatonin is sometimes used in children who have sleep onset insomnia. Restless legs syndrome has been found to be more common in those with ADHD and is often due to iron deficiency anemia. However, restless legs can simply be a part of ADHD and requires careful assessment to differentiate between the two disorders. Delayed sleep phase disorder is also a common comorbidity.
Individuals with ADHD are at increased risk of substance use disorders. This is most commonly seen with alcohol or cannabis. The reason for this may be an altered reward pathway in the brains of ADHD individuals, self-treatment and increased psychosocial risk factors. This makes the evaluation and treatment of ADHD more difficult, with serious substance misuse problems usually treated first due to their greater risks. Other psychiatric conditions include reactive attachment disorder, characterised by a severe inability to appropriately relate socially, and cognitive disengagement syndrome, a distinct attention disorder occurring in 30–50% of ADHD cases as a comorbidity, regardless of the presentation; a subset of cases diagnosed with ADHD-PIP have been found to have CDS instead. Individuals with ADHD are three times more likely to be diagnosed with an eating disorder compared to those without ADHD; conversely, individuals with eating disorders are two times more likely to have ADHD than those without eating disorders.
Trauma
ADHD, trauma, and adverse childhood experiences are also comorbid, which could in part be potentially explained by the similarity in presentation between different diagnoses. The symptoms of ADHD and PTSD can have significant behavioural overlap—in particular, motor restlessness, difficulty concentrating, distractibility, irritability/anger, emotional constriction or dysregulation, poor impulse control, and forgetfulness are common in both. This could result in trauma-related disorders or ADHD being mis-identified as the other. Additionally, traumatic events in childhood are a risk factor for ADHD; they can lead to structural brain changes and the development of ADHD behaviours. Finally, the behavioural consequences of ADHD symptoms cause a higher chance of the individual experiencing trauma (and therefore ADHD leads to a concrete diagnosis of a trauma-related disorder).
Non-psychiatric
See also: Accident-proneness § HypophobiaSome non-psychiatric conditions are also comorbidities of ADHD. This includes epilepsy, a neurological condition characterised by recurrent seizures. There are well established associations between ADHD and obesity, asthma and sleep disorders, and an association with celiac disease. Children with ADHD have a higher risk for migraine headaches, but have no increased risk of tension-type headaches. Children with ADHD may also experience headaches as a result of medication.
A 2021 review reported that several neurometabolic disorders caused by inborn errors of metabolism converge on common neurochemical mechanisms that interfere with biological mechanisms also considered central in ADHD pathophysiology and treatment. This highlights the importance of close collaboration between health services to avoid clinical overshadowing.
In June 2021, Neuroscience & Biobehavioral Reviews published a systematic review of 82 studies that all confirmed or implied elevated accident-proneness in ADHD patients and whose data suggested that the type of accidents or injuries and overall risk changes in ADHD patients over the lifespan. In January 2014, Accident Analysis & Prevention published a meta-analysis of 16 studies examining the relative risk of traffic collisions for drivers with ADHD, finding an overall relative risk estimate of 1.36 without controlling for exposure, a relative risk estimate of 1.29 when controlling for publication bias, a relative risk estimate of 1.23 when controlling for exposure, and a relative risk estimate of 1.86 for ADHD drivers with oppositional defiant disorder and/or conduct disorder comorbidities.
Problematic digital media use
See also: Screen time, Internet addiction disorder, Problematic smartphone use, Problematic social media use, and Video game addiction This section is an excerpt from Digital media use and mental health § ADHD.In April 2018, the International Journal of Environmental Research and Public Health published a systematic review of 24 studies researching associations between internet gaming disorder (IGD) and various psychopathologies that found an 85% correlation between IGD and ADHD. In October 2018, PNAS USA published a systematic review of four decades of research on the relationship between children and adolescents' screen media use and ADHD-related behaviours and concluded that a statistically small relationship between children's media use and ADHD-related behaviours exists. In November 2018, Cyberpsychology published a systematic review and meta-analysis of 5 studies that found evidence for a relationship between problematic smartphone use and impulsivity traits. In October 2020, the Journal of Behavioral Addictions published a systematic review and meta-analysis of 40 studies with 33,650 post-secondary student subjects that found a weak-to-moderate positive association between mobile phone addiction and impulsivity. In January 2021, the Journal of Psychiatric Research published a systematic review of 29 studies including 56,650 subjects that found that ADHD symptoms were consistently associated with gaming disorder and more frequent associations between inattention and gaming disorder than other ADHD scales.
In July 2021, Frontiers in Psychiatry published a meta-analysis reviewing 40 voxel-based morphometry studies and 59 functional magnetic resonance imaging studies comparing subjects with IGD or ADHD to control groups that found that IGD and ADHD subjects had disorder-differentiating structural neuroimage alterations in the putamen and orbitofrontal cortex (OFC) respectively, and functional alterations in the precuneus for IGD subjects and in the rewards circuit (including the OFC, the anterior cingulate cortex, and striatum) for both IGD and ADHD subjects. In March 2022, JAMA Psychiatry published a systematic review and meta-analysis of 87 studies with 159,425 subjects 12 years of age or younger that found a small but statistically significant correlation between screen time and ADHD symptoms in children. In April 2022, Developmental Neuropsychology published a systematic review of 11 studies where the data from all but one study suggested that heightened screen time for children is associated with attention problems. In July 2022, the Journal of Behavioral Addictions published a meta-analysis of 14 studies comprising 2,488 subjects aged 6 to 18 years that found significantly more severe problematic internet use in subjects diagnosed with ADHD to control groups.
In December 2022, European Child & Adolescent Psychiatry published a systematic literature review of 28 longitudinal studies published from 2011 through 2021 of associations between digital media use by children and adolescents and later ADHD symptoms and found reciprocal associations between digital media use and ADHD symptoms (i.e. that subjects with ADHD symptoms were more likely to develop problematic digital media use and that increased digital media use was associated with increased subsequent severity of ADHD symptoms). In May 2023, Reviews on Environmental Health published a meta-analysis of 9 studies with 81,234 child subjects that found a positive correlation between screen time and ADHD risk in children and that higher amounts of screen time in childhood may significantly contribute to the development of ADHD. In December 2023, the Journal of Psychiatric Research published a meta-analysis of 24 studies with 18,859 subjects with a mean age of 18.4 years that found significant associations between ADHD and problematic internet use, while Clinical Psychology Review published a systematic review and meta-analysis of 48 studies examining associations between ADHD and gaming disorder that found a statistically significant association between the disorders.Suicide risk
Systematic reviews in 2017 and 2020 found strong evidence that ADHD is associated with increased suicide risk across all age groups, as well as growing evidence that an ADHD diagnosis in childhood or adolescence represents a significant future suicidal risk factor. Potential causes include ADHD's association with functional impairment, negative social, educational and occupational outcomes, and financial distress. A 2019 meta-analysis indicated a significant association between ADHD and suicidal spectrum behaviours (suicidal attempts, ideations, plans, and completed suicides); across the studies examined, the prevalence of suicide attempts in individuals with ADHD was 18.9%, compared to 9.3% in individuals without ADHD, and the findings were substantially replicated among studies which adjusted for other variables. However, the relationship between ADHD and suicidal spectrum behaviours remains unclear due to mixed findings across individual studies and the complicating impact of comorbid psychiatric disorders. There is no clear data on whether there is a direct relationship between ADHD and suicidality, or whether ADHD increases suicide risk through comorbidities.
Causes
ADHD arises from brain maldevelopment especially in the prefrontal executive networks that can arise either from genetic factors (different gene variants and mutations for building and regulating such networks) or from acquired disruptions to the development of these networks and regions; involved in executive functioning and self-regulation. Their reduced size, functional connectivity, and activation contribute to the pathophysiology of ADHD, as well as imbalances in the noradrenergic and dopaminergic systems that mediate these brain regions.
Genetic factors play an important role; ADHD has a heritability rate of 70-80%. The remaining 20-30% of variance is mediated by de-novo mutations and non-shared environmental factors that provide for or produce brain injuries; there is no significant contribution of the rearing family and social environment. Very rarely, ADHD can also be the result of abnormalities in the chromosomes.
Genetics
See also: Missing heritability problemIn November 1999, Biological Psychiatry published a literature review by psychiatrists Joseph Biederman and Thomas Spencer found the average heritability estimate of ADHD from twin studies to be 0.8, while a subsequent family, twin, and adoption studies literature review published in Molecular Psychiatry in April 2019 by psychologists Stephen Faraone and Henrik Larsson that found an average heritability estimate of 0.74. Additionally, evolutionary psychiatrist Randolph M. Nesse has argued that the 5:1 male-to-female sex ratio in the epidemiology of ADHD suggests that ADHD may be the end of a continuum where males are overrepresented at the tails, citing clinical psychologist Simon Baron-Cohen's suggestion for the sex ratio in the epidemiology of autism as an analogue.
Natural selection has been acting against the genetic variants for ADHD over the course of at least 45,000 years, indicating that it was not an adaptive trait in ancient times. The disorder may remain at a stable rate by the balance of genetic mutations and removal rate (natural selection) across generations; over thousands of years, these genetic variants become more stable, decreasing disorder prevalence. Throughout human evolution, the EFs involved in ADHD likely provide the capacity to bind contingencies across time thereby directing behaviour toward future over immediate events so as to maximise future social consequences for humans.
ADHD has a high heritability of 74%, meaning that 74% of the presence of ADHD in the population is due to genetic factors. There are multiple gene variants which each slightly increase the likelihood of a person having ADHD; it is polygenic and thus arises through the accumulation of many genetic risks each having a very small effect. The siblings of children with ADHD are three to four times more likely to develop the disorder than siblings of children without the disorder.
The association of maternal smoking observed in large population studies disappears after adjusting for family history of ADHD, which indicates that the association between maternal smoking during pregnancy and ADHD is due to familial or genetic factors that increase the risk for the confluence of smoking and ADHD.
ADHD presents with reduced size, functional connectivity and activation as well as low noradrenergic and dopaminergic functioning in brain regions and networks crucial for executive functioning and self-regulation. Typically, a number of genes are involved, many of which directly affect brain functioning and neurotransmission. Those involved with dopamine include DAT, DRD4, DRD5, TAAR1, MAOA, COMT, and DBH. Other genes associated with ADHD include SERT, HTR1B, SNAP25, GRIN2A, ADRA2A, TPH2, and BDNF. A common variant of a gene called latrophilin 3 is estimated to be responsible for about 9% of cases and when this variant is present, people are particularly responsive to stimulant medication. The 7 repeat variant of dopamine receptor D4 (DRD4–7R) causes increased inhibitory effects induced by dopamine and is associated with ADHD. The DRD4 receptor is a G protein-coupled receptor that inhibits adenylyl cyclase. The DRD4–7R mutation results in a wide range of behavioural phenotypes, including ADHD symptoms reflecting split attention. The DRD4 gene is both linked to novelty seeking and ADHD. The genes GFOD1 and CDH13 show strong genetic associations with ADHD. CDH13's association with ASD, schizophrenia, bipolar disorder, and depression make it an interesting candidate causative gene. Another candidate causative gene that has been identified is ADGRL3. In zebrafish, knockout of this gene causes a loss of dopaminergic function in the ventral diencephalon and the fish display a hyperactive/impulsive phenotype.
For genetic variation to be used as a tool for diagnosis, more validating studies need to be performed. However, smaller studies have shown that genetic polymorphisms in genes related to catecholaminergic neurotransmission or the SNARE complex of the synapse can reliably predict a person's response to stimulant medication. Rare genetic variants show more relevant clinical significance as their penetrance (the chance of developing the disorder) tends to be much higher. However their usefulness as tools for diagnosis is limited as no single gene predicts ADHD. ASD shows genetic overlap with ADHD at both common and rare levels of genetic variation.
Environment
In addition to genetics, some environmental factors might play a role in causing ADHD. Alcohol intake during pregnancy can cause fetal alcohol spectrum disorders which can include ADHD or symptoms like it. Children exposed to certain toxic substances, such as lead or polychlorinated biphenyls, may develop problems which resemble ADHD. Exposure to the organophosphate insecticides chlorpyrifos and dialkyl phosphate is associated with an increased risk; however, the evidence is not conclusive. Exposure to tobacco smoke during pregnancy can cause problems with central nervous system development and can increase the risk of ADHD. Nicotine exposure during pregnancy may be an environmental risk.
Extreme premature birth, very low birth weight, and extreme neglect, abuse, or social deprivation also increase the risk as do certain infections during pregnancy, at birth, and in early childhood. These infections include, among others, various viruses (measles, varicella zoster encephalitis, rubella, enterovirus 71). At least 30% of children with a traumatic brain injury later develop ADHD and about 5% of cases are due to brain damage.
Some studies suggest that in a small number of children, artificial food dyes or preservatives may be associated with an increased prevalence of ADHD or ADHD-like symptoms, but the evidence is weak and may apply to only children with food sensitivities. The European Union has put in place regulatory measures based on these concerns. In a minority of children, intolerances or allergies to certain foods may worsen ADHD symptoms.
Individuals with hypokalemic sensory overstimulation are sometimes diagnosed as having ADHD, raising the possibility that a subtype of ADHD has a cause that can be understood mechanistically and treated in a novel way. The sensory overload is treatable with oral potassium gluconate.
Research does not support popular beliefs that ADHD is caused by eating too much refined sugar, watching too much television, bad parenting, poverty or family chaos; however, they might worsen ADHD symptoms in certain people.
In some cases, an inappropriate diagnosis of ADHD may reflect a dysfunctional family or a poor educational system, rather than any true presence of ADHD in the individual. In other cases, it may be explained by increasing academic expectations, with a diagnosis being a method for parents in some countries to obtain extra financial and educational support for their child. Behaviours typical of ADHD occur more commonly in children who have experienced violence and emotional abuse.
Pathophysiology
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems, particularly those involving dopamine and norepinephrine. The dopamine and norepinephrine pathways that originate in the ventral tegmental area and locus coeruleus project to diverse regions of the brain and govern a variety of cognitive processes. The dopamine pathways and norepinephrine pathways which project to the prefrontal cortex and striatum are directly responsible for modulating executive function (cognitive control of behaviour), motivation, reward perception, and motor function; these pathways are known to play a central role in the pathophysiology of ADHD. Larger models of ADHD with additional pathways have been proposed.
Brain structure

In children with ADHD, there is a general reduction of volume in certain brain structures, with a proportionally greater decrease in the volume in the left-sided prefrontal cortex. The posterior parietal cortex also shows thinning in individuals with ADHD compared to controls. Other brain structures in the prefrontal-striatal-cerebellar and prefrontal-striatal-thalamic circuits have also been found to differ between people with and without ADHD.
The subcortical volumes of the accumbens, amygdala, caudate, hippocampus, and putamen appears smaller in individuals with ADHD compared with controls. Structural MRI studies have also revealed differences in white matter, with marked differences in inter-hemispheric asymmetry between ADHD and typically developing youths.
Functional MRI (fMRI) studies have revealed a number of differences between ADHD and control brains. Mirroring what is known from structural findings, fMRI studies have showed evidence for a higher connectivity between subcortical and cortical regions, such as between the caudate and prefrontal cortex. The degree of hyperconnectivity between these regions correlated with the severity of inattention or hyperactivity Hemispheric lateralization processes have also been postulated as being implicated in ADHD, but empiric results showed contrasting evidence on the topic.
Neurotransmitter pathways
Previously, it had been suggested that the elevated number of dopamine transporters in people with ADHD was part of the pathophysiology, but it appears the elevated numbers may be due to adaptation following exposure to stimulant medication. Current models involve the mesocorticolimbic dopamine pathway and the locus coeruleus-noradrenergic system. ADHD psychostimulants possess treatment efficacy because they increase neurotransmitter activity in these systems. There may additionally be abnormalities in serotonergic, glutamatergic, or cholinergic pathways.
Executive function and motivation
ADHD arises from a core deficit in executive functions (e.g., attentional control, inhibitory control, and working memory), which are a set of cognitive processes that are required to successfully select and monitor behaviours that facilitate the attainment of one's chosen goals. The executive function impairments that occur in ADHD individuals result in problems with staying organised, time keeping, procrastination control, maintaining concentration, paying attention, ignoring distractions, regulating emotions, and remembering details. People with ADHD appear to have unimpaired long-term memory, and deficits in long-term recall appear to be attributed to impairments in working memory. Due to the rates of brain maturation and the increasing demands for executive control as a person gets older, ADHD impairments may not fully manifest themselves until adolescence or even early adulthood. Conversely, brain maturation trajectories, potentially exhibiting diverging longitudinal trends in ADHD, may support a later improvement in executive functions after reaching adulthood.
ADHD has also been associated with motivational deficits in children. Children with ADHD often find it difficult to focus on long-term over short-term rewards, and exhibit impulsive behaviour for short-term rewards.
Paradoxical reaction to neuroactive substances
Another sign of the structurally altered signal processing in the central nervous system in this group of people is the conspicuously common paradoxical reaction (c. 10–20% of patients). These are unexpected reactions in the opposite direction as with a normal effect, or otherwise significant different reactions. These are reactions to neuroactive substances such as local anesthetic at the dentist, sedative, caffeine, antihistamine, weak neuroleptics and central and peripheral painkillers. Since the causes of paradoxical reactions are at least partly genetic, it may be useful in critical situations, for example before operations, to ask whether such abnormalities may also exist in family members.
Diagnosis
ADHD is diagnosed by an assessment of a person's behavioural and mental development, including ruling out the effects of drugs, medications, and other medical or psychiatric problems as explanations for the symptoms. ADHD diagnosis often takes into account feedback from parents and teachers with most diagnoses begun after a teacher raises concerns. While many tools exist to aid in the diagnosis of ADHD, their validity varies in different populations, and a reliable and valid diagnosis requires confirmation by a clinician while supplemented by standardized rating scales and input from multiple informants across various settings. The diagnosis of ADHD has been criticised as being subjective because it is not based on a biological test. The International Consensus Statement on ADHD concluded that this criticism is unfounded, on the basis that ADHD meets standard criteria for validity of a mental disorder established by Robins and Guze. They attest that the disorder is considered valid because: 1) well-trained professionals in a variety of settings and cultures agree on its presence or absence using well-defined criteria and 2) the diagnosis is useful for predicting a) additional problems the patient may have (e.g., difficulties learning in school); b) future patient outcomes (e.g., risk for future drug abuse); c) response to treatment (e.g., medications and psychological treatments); and d) features that indicate a consistent set of causes for the disorder (e.g., findings from genetics or brain imaging), and that professional associations have endorsed and published guidelines for diagnosing ADHD.
The most commonly used rating scales for diagnosing ADHD are the Achenbach System of Empirically Based Assessment (ASEBA) and include the Child Behavior Checklist (CBCL) used for parents to rate their child's behaviour, the Youth Self Report Form (YSR) used for children to rate their own behaviour, and the Teacher Report Form (TRF) used for teachers to rate their pupil's behaviour. Additional rating scales that have been used alone or in combination with other measures to diagnose ADHD include the Behavior Assessment System for Children (BASC), Behavior Rating Inventory of Executive Function - Second Edition (BRIEF2), Revised Conners Rating Scale (CRS-R), Conduct-Hyperactive-Attention Problem-Oppositional Symptom scale (CHAOS), Developmental Behavior Checklist Hyperactivity Index (DBC-HI), Parent Disruptive Behavior Disorder Ratings Scale (DBDRS), Diagnostic Infant and Preschool Assessment (DIPA-L), Pediatric Symptom Checklist (PSC), Social Communication Questionnaire (SCQ), Social Responsiveness Scale (SRS), Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Rating Scale (SWAN). and the Vanderbilt ADHD diagnostic rating scale.
The ASEBA, BASC, CHAOS, CRS, and Vanderbilt diagnostic rating scales allow for both parents and teachers as raters in the diagnosis of childhood and adolescent ADHD. Adolescents may also self report their symptoms using self report scales from the ASEBA, SWAN, and the Dominic Interactive for Adolescents-Revised (DIA-R). Self-rating scales, such as the ADHD rating scale and the Vanderbilt ADHD diagnostic rating scale, are used in the screening and evaluation of ADHD.
Based on a 2024 systematic literature review and meta analysis commissioned by the Patient-Centered Outcomes Research Institute (PCORI), rating scales based on parent report, teacher report, or self-assessment from the adolescent have high internal consistency as a diagnostic tool meaning that the items within the scale are highly interrelated. The reliability of the scales between raters (i.e. their degree of agreement) however is poor to moderate making it important to include information from multiple raters to best inform a diagnosis.
Imaging studies of the brain do not give consistent results between individuals; thus, they are only used for research purposes and not a diagnosis. Electroencephalography is not accurate enough to make an ADHD diagnosis. A 2024 systematic review concluded that the use of biomarkers such as blood or urine samples, electroencephalogram (EEG) markers, and neuroimaging such as MRIs, in diagnosis for ADHD remains unclear; studies showed great variability, did not assess test-retest reliability, and were not independently replicable.
In North America and Australia, DSM-5 criteria are used for diagnosis, while European countries usually use the ICD-10. The DSM-IV criteria for diagnosis of ADHD is 3–4 times more likely to diagnose ADHD than is the ICD-10 criteria. ADHD is alternately classified as neurodevelopmental disorder or a disruptive behaviour disorder along with ODD, CD, and antisocial personality disorder. A diagnosis does not imply a neurological disorder.
Very few studies have been conducted on diagnosis of ADHD on children younger than 7 years of age, and those that have were found in a 2024 systematic review to be of low or insufficient strength of evidence.
Classification
Diagnostic and Statistical Manual
As with many other psychiatric disorders, a formal diagnosis should be made by a qualified professional based on a set number of criteria. In the United States, these criteria are defined by the American Psychiatric Association in the DSM. Based on the DSM-5 criteria published in 2013 and the DSM-5-TR criteria published in 2022, there are three presentations of ADHD:
- ADHD, predominantly inattentive presentation, presents with symptoms including being easily distracted, forgetful, daydreaming, disorganization, poor sustained attention, and difficulty completing tasks.
- ADHD, predominantly hyperactive-impulsive presentation, presents with excessive fidgeting and restlessness, hyperactivity, and difficulty waiting and remaining seated.
- ADHD, combined presentation, is a combination of the first two presentations.
This subdivision is based on presence of at least six (in children) or five (in older teenagers and adults) out of nine long-term (lasting at least six months) symptoms of inattention, hyperactivity–impulsivity, or both. To be considered, several symptoms must have appeared by the age of six to twelve and occur in more than one environment (e.g. at home and at school or work). The symptoms must be inappropriate for a child of that age and there must be clear evidence that they are causing impairment in multiple domains of life.
The DSM-5 and the DSM-5-TR also provide two diagnoses for individuals who have symptoms of ADHD but do not entirely meet the requirements. Other Specified ADHD allows the clinician to describe why the individual does not meet the criteria, whereas Unspecified ADHD is used where the clinician chooses not to describe the reason.
International Classification of Diseases
In the eleventh revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-11) by the World Health Organization, the disorder is classified as Attention deficit hyperactivity disorder (code 6A05). The defined subtypes are predominantly inattentive presentation (6A05.0); predominantly hyperactive-impulsive presentation(6A05.1); and combined presentation (6A05.2). However, the ICD-11 includes two residual categories for individuals who do not entirely match any of the defined subtypes: other specified presentation (6A05.Y) where the clinician includes detail on the individual's presentation; and presentation unspecified (6A05.Z) where the clinician does not provide detail.
In the tenth revision (ICD-10), the symptoms of hyperkinetic disorder were analogous to ADHD in the ICD-11. When a conduct disorder (as defined by ICD-10) is present, the condition was referred to as hyperkinetic conduct disorder. Otherwise, the disorder was classified as disturbance of activity and attention, other hyperkinetic disorders or hyperkinetic disorders, unspecified. The latter was sometimes referred to as hyperkinetic syndrome.
Social construct theory
The social construct theory of ADHD suggests that, because the boundaries between normal and abnormal behaviour are socially constructed (i.e. jointly created and validated by all members of society, and in particular by physicians, parents, teachers, and others), it then follows that subjective valuations and judgements determine which diagnostic criteria are used and thus, the number of people affected. Thomas Szasz, a supporter of this theory, has argued that ADHD was "invented and then given a name".
Adults
Main article: Adult attention deficit hyperactivity disorderAdults with ADHD are diagnosed under the same criteria, including that their signs must have been present by the age of six to twelve. The individual is the best source for information in diagnosis, however others may provide useful information about the individual's symptoms currently and in childhood; a family history of ADHD also adds weight to a diagnosis. While the core symptoms of ADHD are similar in children and adults, they often present differently in adults than in children: for example, excessive physical activity seen in children may present as feelings of restlessness and constant mental activity in adults.
Worldwide, it is estimated that 2.58% of adults have persistent ADHD (where the individual currently meets the criteria and there is evidence of childhood onset), and 6.76% of adults have symptomatic ADHD (meaning that they currently meet the criteria for ADHD, regardless of childhood onset). In 2020, this was 139.84 million and 366.33 million affected adults respectively. Around 15% of children with ADHD continue to meet full DSM-IV-TR criteria at 25 years of age, and 50% still experience some symptoms. As of 2010, most adults remain untreated. Many adults with ADHD without diagnosis and treatment have a disorganised life, and some use non-prescribed drugs or alcohol as a coping mechanism. Other problems may include relationship and job difficulties, and an increased risk of criminal activities. Associated mental health problems include depression, anxiety disorders, and learning disabilities.
Some ADHD symptoms in adults differ from those seen in children. While children with ADHD may climb and run about excessively, adults may experience an inability to relax, or may talk excessively in social situations. Adults with ADHD may start relationships impulsively, display sensation-seeking behaviour, and be short-tempered. Addictive behaviour such as substance abuse and gambling are common. This led to those who presented differently as they aged having outgrown the DSM-IV criteria. The DSM-5 criteria does specifically deal with adults unlike that of DSM-IV, which does not fully take into account the differences in impairments seen in adulthood compared to childhood.
For diagnosis in an adult, having symptoms since childhood is required. Nevertheless, a proportion of adults who meet the criteria for ADHD in adulthood would not have been diagnosed with ADHD as children. Most cases of late-onset ADHD develop the disorder between the ages of 12–16 and may therefore be considered early adult or adolescent-onset ADHD.
Differential diagnosis
| Depressive disorder | Anxiety disorder | Bipolar disorder |
|---|---|---|
|
|
in manic state
in depressive state
|
The DSM provides differential diagnoses – potential alternate explanations for specific symptoms. Assessment and investigation of clinical history determines which is the most appropriate diagnosis. The DSM-5 suggests oppositional defiant disorder, intermittent explosive disorder, and other disorders such as stereotypic movement disorder and Tourette syndrome, in addition to specific learning disorder, intellectual disability, autism, reactive attachment disorder, anxiety disorders, depressive disorders, bipolar disorder, disruptive mood dysregulation disorder, substance use disorder, personality disorders, psychotic disorders, medication-induced symptoms, and neurocognitive disorders. Many but not all of these are also common comorbidities of ADHD. The DSM-5-TR also suggests post-traumatic stress disorder.
Symptoms of ADHD that particularly relate to disinhibition and irritability in addition to low-mood and self-esteem as a result of symptom expression might be confusable with dysthymia and bipolar disorder as well as with borderline personality disorder, however they are comorbid at a significantly increased rate relative to the general population. Some symptoms that are viewed superficially due to anxiety disorders, intellectual disability or the effects of substance abuse such as intoxication and withdrawal can overlap to some extent with ADHD. These disorders can also sometimes occur along with ADHD.
Primary sleep disorders may affect attention and behaviour and the symptoms of ADHD may affect sleep. It is thus recommended that children with ADHD be regularly assessed for sleep problems. Sleepiness in children may result in symptoms ranging from the classic ones of yawning and rubbing the eyes, to disinhibition and inattention. Obstructive sleep apnea can also cause ADHD-like symptoms.
In general, the DSM-5-TR can help distinguish between many conditions associated with ADHD-like symptoms by the context in which the symptoms arise. For example, children with learning disabilities may feel distractable and agitated when asked to engage in tasks that require the impaired skill (e.g., reading, math), but not in other situations. A person with an intellectual disability may develop symptoms that overlap with ADHD when placed in a school environment that is inappropriate for their needs. The type of inattention implicated in ADHD, of poor persistence and sustained attention, differs substantially from selective or oriented inattention seen in cognitive disengagement syndrome (CDS), as well as from rumination, reexperiencing or mind blanking seen in anxiety disorders or PTSD.
In mood disorders, ADHD-like symptoms may be limited to manic or depressive states of an episodic nature. Symptoms overlapping with ADHD in psychotic disorders may be limited to psychotic states. Substance use disorder, some medications, and certain medical conditions may cause symptoms to appear later in life, while ADHD, as a neurodevelopmental disorder, requires for them to have been present since childhood.
Furthermore, a careful understanding of the nature of the symptoms may help establish the difference between ADHD and other disorders. For example, the forgetfulness and impulsivity typical of ADHD (e.g., in completing school assignments or following directions) may be distinguished from opposition when there is no hostility or defiance, although ADHD and ODD are highly comorbid. Tantrums may differ from the outbursts in intermittent explosive disorder if there is no aggression involved. The fidgetiness observed in ADHD may be differentiated from tics or stereotypies common in Tourette's disorder or autism.
Also, the social difficulties often experienced by individuals with ADHD due to inattention (e.g., being unfocused during the interaction and therefore missing cues or being unaware of one's behavior) or impulsivity (blurting things out, asking intrusive questions, interrupting) may be contrasted with the social detachment and deficits in understanding social cues associated with autism. Individuals with ADHD may also present signs of the social impairment or emotional and cognitive dysregulation seen in personality disorders, but not necessarily such features as a fear of abandonment, an unstable sense of self, narcissistic tendencies, aggressiveness, or other personality features.
While it is possible and common for many of these different conditions to be comorbid with ADHD, the symptoms must not be better explained by them, as per diagnostic criterion E in the DSM-5. The symptoms must arise early in life, appear across multiple environments, and cause significant impairment. Moreover, when some of these conditions are in fact comorbid with ADHD, it is still important to distinguish them, as each may need to be treated separately.
Management
Main article: Attention deficit hyperactivity disorder managementThe management of ADHD typically involves counseling or medications, either alone or in combination. While there are various options of treatment to improve ADHD symptoms, medication therapies substantially improve long-term outcomes, and while eliminating some elevated risks such as obesity, they do come with some risks of adverse events. Medications used include stimulants, atomoxetine, alpha-2 adrenergic receptor agonists, and sometimes antidepressants. In those who have trouble focusing on long-term rewards, a large amount of positive reinforcement improves task performance. Medications are the most effective treatment, and any side effects are typically mild and easy to resolve although any improvements will be reverted if medication is ceased. ADHD stimulants also improve persistence and task performance in children with ADHD. To quote one systematic review, "recent evidence from observational and registry studies indicates that pharmacological treatment of ADHD is associated with increased achievement and decreased absenteeism at school, a reduced risk of trauma-related emergency hospital visits, reduced risks of suicide and attempted suicide, and decreased rates of substance abuse and criminality". Data also suggest that combining medication with cognitive behavioral therapy (CBT) can have positive effects: although CBT is substantially less effective, it can help address problems that reside after medication has been optimised. The nature and range of desirable endpoints of ADHD treatment vary among diagnostic standards for ADHD. In most studies, the efficacy of treatment is determined by reductions in symptoms. However, some studies have included subjective ratings from teachers and parents as part of their assessment of treatment efficacies.
Behavioural therapies
There is good evidence for the use of behavioural therapies in ADHD. They are the recommended first-line treatment in those who have mild symptoms or who are preschool-aged. Psychological therapies used include: psychoeducational input, behavior therapy, cognitive behavioral therapy, interpersonal psychotherapy, family therapy, school-based interventions, social skills training, behavioural peer intervention, organization training, and parent management training. Neurofeedback has greater treatment effects than non-active controls for up to 6 months and possibly a year following treatment, and may have treatment effects comparable to active controls (controls proven to have a clinical effect) over that time period. Despite efficacy in research, there is insufficient regulation of neurofeedback practice, leading to ineffective applications and false claims regarding innovations. Parent training may improve a number of behavioural problems including oppositional and non-compliant behaviours.
There is little high-quality research on the effectiveness of family therapy for ADHD—but the existing evidence shows that it is similar to community care, and better than placebo. ADHD-specific support groups can provide information and may help families cope with ADHD.
Social skills training, behavioural modification, and medication may have some limited beneficial effects in peer relationships. Stable, high-quality friendships with non-deviant peers protect against later psychological problems.
Digital interventions
Several clinical trials have investigated the efficacy of digital therapeutics, particularly Akili Interactive Labs's video game-based digital therapeutic AKL-T01, marketed as EndeavourRx. The pediatric STARS-ADHD randomized, double-blind, parallel-group, controlled trial demonstrated that AKL-T01 significantly improved performance on the Test of Variables of Attention, an objective measure of attention and inhibitory control, compared to a control group after four weeks of at-home use. A subsequent pediatric open-label study, STARS-Adjunct, published in Nature Portfolio's npj Digital Medicine evaluated AKL-T01 as an adjunctive treatment for children with ADHD who were either on stimulant medication or not on stimulant pharmacotherapy. Results showed improvements in ADHD-related impairment (measured by the Impairment Rating Scale) and ADHD symptoms after 4 weeks of treatment, with effects persisting during a 4-week pause and further improving with an additional treatment period. Notably, the magnitude of the measured improvement was similar for children both on and off stimulants. In 2020, AKL-T01 received marketing authorization for pediatric ADHD from the FDA, becoming "the first game-based therapeutic granted marketing authorization by the FDA for any type of condition."
In addition to pediatric populations, a 2023 study in the Journal of the American Academy of Child & Adolescent Psychiatry investigated the efficacy and safety of AKL-T01 in adults with ADHD. After six weeks of at-home treatment with AKL-T01, participants showed significant improvements in objective measures of attention (TOVA - Attention Comparison Score), reported ADHD symptoms (ADHD-RS-IV inattention subscale and total score), and reported quality of life (AAQoL). The magnitude of improvement in attention was nearly seven times greater than that reported in pediatric trials. The treatment was well-tolerated, with high compliance and no serious adverse events.
Medication
The medications for ADHD appear to alleviate symptoms via their effects on the pre-frontal executive, striatal and related regions and networks in the brain; usually by increasing neurotransmission of norepinephrine and dopamine.
Stimulants
Methylphenidate and amphetamine or its derivatives are often first-line treatments for ADHD. About 70 per cent respond to the first stimulant tried and as few as 10 per cent respond to neither amphetamines nor methylphenidate. Stimulants may also reduce the risk of unintentional injuries in children with ADHD. Magnetic resonance imaging studies suggest that long-term treatment with amphetamine or methylphenidate decreases abnormalities in brain structure and function found in subjects with ADHD. A 2018 review found the greatest short-term benefit with methylphenidate in children, and amphetamines in adults. Studies and meta-analyses show that amphetamine is slightly-to-modestly more effective than methylphenidate at reducing symptoms, and they are more effective pharmacotherapy for ADHD than α2-agonists but methylphenidate has comparable efficacy to non-stimulants such as atomoxetine. In a Cochrane clinical synopsis, Dr Storebø and colleagues summarised their meta-review on methylphenidate for ADHD in children and adolescents. The meta-analysis raised substantial doubts about the drug's efficacy relative to a placebo. This led to a strong critical reaction from the European ADHD Guidelines Group and individuals in the scientific community, who identified a number of flaws in the review. Since at least September 2021, there is a unanimous and global scientific consensus that methylphenidate is safe and highly effective for treating ADHD. The same journal released a subsequent systematic review (2022) of extended-release methylphenidate for adults, concluding similar doubts about the certainty of evidence. Other recent systematic reviews and meta-analyses, however, find certainty in the safety and high efficacy of methylphenidate for reducing ADHD symptoms, for alleviating the underlying executive functioning deficits, and for substantially reducing the adverse consequences of untreated ADHD with continuous treatment. Clinical guidelines internationally are also consistent in approving the safety and efficacy of methylphenidate and recommending it as a first-line treatment for the disorder.
Safety and efficacy data have been reviewed extensively by medical regulators (e.g., the US Food and Drug Administration and the European Medicines Agency), the developers of evidence-based international guidelines (e.g., the UK National Institute for Health and Care Excellence and the American Academy of Pediatrics), and government agencies who have endorsed these guidelines (e.g., the Australian National Health and Medical Research Council). These professional groups unanimously conclude, based on the scientific evidence, that methylphenidate is safe and effective and should be considered as a first-line treatment for ADHD. The likelihood of developing insomnia for ADHD patients taking stimulants has been measured at between 11 and 45 per cent for different medications, and may be a main reason for discontinuation. Other side effects, such as tics, decreased appetite and weight loss, or emotional lability, may also lead to discontinuation. Stimulant psychosis and mania are rare at therapeutic doses, appearing to occur in approximately 0.1% of individuals, within the first several weeks after starting amphetamine therapy. The safety of these medications in pregnancy is unclear. Symptom improvement is not sustained if medication is ceased.
The long-term effects of ADHD medication have yet to be fully determined, although stimulants are generally beneficial and safe for up to two years for children and adolescents. A 2022 meta-analysis found no statistically significant association between ADHD medications and the risk of cardiovascular disease (CVD) across age groups, although the study suggests further investigation is warranted for patients with preexisting CVD as well as long-term medication use. Regular monitoring has been recommended in those on long-term treatment. There are indications suggesting that stimulant therapy for children and adolescents should be stopped periodically to assess continuing need for medication, decrease possible growth delay, and reduce tolerance. Although potentially addictive at high doses, stimulants used to treat ADHD have low potential for abuse. Treatment with stimulants is either protective against substance abuse or has no effect.
The majority of studies on nicotine and other nicotinic agonists as treatments for ADHD have shown favorable results; however, no nicotinic drug has been approved for ADHD treatment. Caffeine was formerly used as a second-line treatment for ADHD but research indicates it has no significant effects in reducing ADHD symptoms. Caffeine appears to help with alertness, arousal and reaction time but not the type of inattention implicated in ADHD (sustained attention/persistence). Pseudoephedrine and ephedrine do not affect ADHD symptoms.
Modafinil has shown some efficacy in reducing the severity of ADHD in children and adolescents. It may be prescribed off-label to treat ADHD.
Non-stimulants
Two non-stimulant medications, atomoxetine and viloxazine, are approved by the FDA and in other countries for the treatment of ADHD.
Atomoxetine, due to its lack of addiction liability, may be preferred in those who are at risk of recreational or compulsive stimulant use, although evidence is lacking to support its use over stimulants for this reason. Atomoxetine alleviates ADHD symptoms through norepinephrine reuptake and by indirectly increasing dopamine in the pre-frontal cortex, sharing 70-80% of the brain regions with stimulants in their produced effects. Atomoxetine has been shown to significantly improve academic performance. Meta-analyses and systematic reviews have found that atomoxetine has comparable efficacy, equal tolerability and response rate (75%) to methylphenidate in children and adolescents. In adults, efficacy and discontinuation rates are equivalent.
Analyses of clinical trial data suggests that viloxazine is about as effective as atomoxetine and methylphenidate but with fewer side effects.
Amantadine was shown to induce similar improvements in children treated with methylphenidate, with less frequent side effects. A 2021 retrospective study showed that amantadine may serve as an effective adjunct to stimulants for ADHD–related symptoms and appears to be a safer alternative to second- or third-generation antipsychotics.
Bupropion is also used off-label by some clinicians due to research findings. It is effective, but modestly less than atomoxetine and methylphenidate.
There is little evidence on the effects of medication on social behaviours. Antipsychotics may also be used to treat aggression in ADHD.
Alpha-2a agonists
Two alpha-2a agonists, extended-release formulations of guanfacine and clonidine, are approved by the FDA and in other countries for the treatment of ADHD (effective in children and adolescents but effectiveness has still not been shown for adults). They appear to be modestly less effective than the stimulants (amphetamine and methylphenidate) and non-stimulants (atomoxetine and viloxazine) at reducing symptoms, but can be useful alternatives or used in conjunction with a stimulant. These medications act by adjusting the alpha-2a ports on the outside of noradrenergic nerve cells in the pre-frontal executive networks, so the information (electrical signal) is less confounded by noise.
Guidelines
Guidelines on when to use medications vary by country. The United Kingdom's National Institute for Health and Care Excellence recommends use for children only in severe cases, though for adults medication is a first-line treatment. Conversely, most United States guidelines recommend medications in most age groups. Medications are especially not recommended for preschool children. Underdosing of stimulants can occur, and can result in a lack of response or later loss of effectiveness. This is particularly common in adolescents and adults as approved dosing is based on school-aged children, causing some practitioners to use weight-based or benefit-based off-label dosing instead.
Exercise
Exercise does not reduce the symptoms of ADHD. The conclusion by the International Consensus Statement is based on two meta-analyses: one of 10 studies with 300 children and the other of 15 studies and 668 participants, which showed that exercise yields no statistically significant reductions on ADHD symptoms. A 2024 systematic review and meta analysis commissioned by the Patient-Centered Outcomes Research Institute (PCORI) identified seven studies on the effectiveness of physical exercise for treating ADHD symptoms. The type and amount of exercise varied widely across studies from martial arts interventions to treadmill training, to table tennis or aerobic exercise. Effects reported were not replicated, causing the authors to conclude that there is insufficient evidence that exercise intervention is an effective form of treatment for ADHD symptoms.
Diet
Dietary modifications are not recommended as of 2019 by the American Academy of Pediatrics, the National Institute for Health and Care Excellence, or the Agency for Healthcare Research and Quality due to insufficient evidence. A 2013 meta-analysis found less than a third of children with ADHD see some improvement in symptoms with free fatty acid supplementation or decreased consumption of artificial food colouring. These benefits may be limited to children with food sensitivities or those who are simultaneously being treated with ADHD medications. This review also found that evidence does not support removing other foods from the diet to treat ADHD. A 2014 review found that an elimination diet results in a small overall benefit in a minority of children, such as those with allergies. A 2016 review stated that the use of a gluten-free diet as standard ADHD treatment is not advised. A 2017 review showed that a few-foods elimination diet may help children too young to be medicated or not responding to medication, while free fatty acid supplementation or decreased eating of artificial food colouring as standard ADHD treatment is not advised. Chronic deficiencies of iron, magnesium and iodine may have a negative impact on ADHD symptoms. There is a small amount of evidence that lower tissue zinc levels may be associated with ADHD. In the absence of a demonstrated zinc deficiency (which is rare outside of developing countries), zinc supplementation is not recommended as treatment for ADHD. However, zinc supplementation may reduce the minimum effective dose of amphetamine when it is used with amphetamine for the treatment of ADHD.
Prognosis
ADHD persists into adulthood in about 30–50% of cases. Those affected are likely to develop coping mechanisms as they mature, thus compensating to some extent for their previous symptoms. Children with ADHD have a higher risk of unintentional injuries. Effects of medication on functional impairment and quality of life (e.g. reduced risk of accidents) have been found across multiple domains. Rates of smoking among those with ADHD are higher than in the general population at about 40%. About 30–50% of people diagnosed in childhood continue to have ADHD in adulthood, with 2.58% of adults estimated to have ADHD which began in childhood. In adults, hyperactivity is usually replaced by inner restlessness, and adults often develop coping skills to compensate for their impairments. The condition can be difficult to tell apart from other conditions, as well as from high levels of activity within the range of normal behaviour. ADHD has a negative impact on patient health-related quality of life that may be further exacerbated by, or may increase the risk of, other psychiatric conditions such as anxiety and depression. Individuals with ADHD may also face misconceptions and stigma.
Individuals with ADHD are significantly overrepresented in prison populations. Although there is no generally accepted estimate of ADHD prevalence among inmates, a 2015 meta-analysis estimated a prevalence of 25.5%, and a larger 2018 meta-analysis estimated the frequency to be 26.2%.
Epidemiology
Main article: Epidemiology of attention deficit hyperactive disorder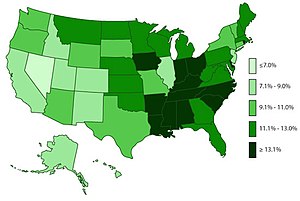
ADHD is estimated to affect about 6–7% of people aged 18 and under when diagnosed via the DSM-IV criteria. When diagnosed via the ICD-10 criteria, rates in this age group are estimated around 1–2%. Rates are similar between countries and differences in rates depend mostly on how it is diagnosed. Children in North America appear to have a higher rate of ADHD than children in Africa and the Middle East; this is believed to be due to differing methods of diagnosis rather than a difference in underlying frequency. (The same publication which describes this difference also notes that the difference may be rooted in the available studies from these respective regions, as far more studies were from North America than from Africa and the Middle East.) As of 2019, it was estimated to affect 84.7 million people globally.
ADHD is diagnosed approximately twice as often in boys as in girls, and 1.6 times more often in men than in women, although the disorder is overlooked in girls or diagnosed in later life because their symptoms sometimes differ from diagnostic criteria. In 2014, Keith Conners, one of the early advocates for recognition of the disorder, spoke out against overdiagnosis in a New York Times article. In contrast, a 2014 peer-reviewed medical literature review indicated that ADHD is underdiagnosed in adults.
Studies from multiple countries have reported that children born closer to the start of the school year are more frequently diagnosed with and medicated for ADHD than their older classmates. Boys who were born in December where the school age cut-off was 31 December were shown to be 30% more likely to be diagnosed and 41% more likely to be treated than those born in January. Girls born in December had a diagnosis and treatment percentage increase of 70% and 77% respectively compared to those born in January. Children who were born at the last three days of a calendar year were reported to have significantly higher levels of diagnosis and treatment for ADHD than children born at the first three days of a calendar year. The studies suggest that ADHD diagnosis is prone to subjective analysis.
Rates of diagnosis and treatment have increased in both the United Kingdom and the United States since the 1970s. Prior to 1970, it was rare for children to be diagnosed with ADHD, while in the 1970s rates were about 1%. This is believed to be primarily due to changes in how the condition is diagnosed and how readily people are willing to treat it with medications rather than a true change in incidence. With widely differing rates of diagnosis across countries, states within countries, races, and ethnicities, some suspect factors other than symptoms of ADHD are playing a role in diagnosis, such as cultural norms.
Despite showing a higher frequency of symptoms associated with ADHD, non-White children in the US are less likely than White children to be diagnosed or treated for ADHD, a finding that is often explained by bias among health professionals, as well as parents who may be reluctant to acknowledge that their child has ADHD. Crosscultural differences in diagnosis of ADHD can also be attributed to the long-lasting effects of harmful, racially targeted medical practices. Medical pseudosciences, particularly those that targeted Black populations during the period of slavery in the US, lead to a distrust of medical practices within certain communities. The combination of ADHD symptoms often being regarded as misbehaviour rather than as a psychiatric condition, and the use of drugs to regulate ADHD, result in a hesitancy to trust a diagnosis of ADHD. Cases of misdiagnosis in ADHD can also occur due to stereotyping of people of color. Due to ADHD's subjectively determined symptoms, medical professionals may diagnose individuals based on stereotyped behaviour or misdiagnose due to cultural differences in symptom presentation.
A 2024 study in CDC’s Morbidity and Mortality Weekly Report reports around 15.5 million U.S. adults have attention-deficit hyperactivity disorder, with many facing challenges in accessing treatment. One-third of diagnosed individuals had received a prescription for a stimulant drug in the past year but nearly three-quarters of them reported difficulties filling the prescription due to medication shortages.
History
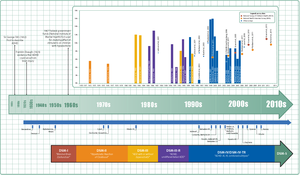
ADHD was officially known as attention deficit disorder (ADD) from 1980 to 1987; prior to the 1980s, it was known as hyperkinetic reaction of childhood. Symptoms similar to those of ADHD have been described in medical literature dating back to the 18th century. Sir Alexander Crichton describes "mental restlessness" in his book An inquiry into the nature and origin of mental derangement written in 1798. He made observations about children showing signs of being inattentive and having the "fidgets". The first clear description of ADHD is credited to George Still in 1902 during a series of lectures he gave to the Royal College of Physicians of London.
The terminology used to describe the condition has changed over time and has included: minimal brain dysfunction in the DSM-I (1952), hyperkinetic reaction of childhood in the DSM-II (1968), and attention-deficit disorder with or without hyperactivity in the DSM-III (1980). In 1987, this was changed to ADHD in the DSM-III-R, and in 1994 the DSM-IV in split the diagnosis into three subtypes: ADHD inattentive type, ADHD hyperactive-impulsive type, and ADHD combined type. These terms were kept in the DSM-5 in 2013 and in the DSM-5-TR in 2022. Prior to the DSM, terms included minimal brain damage in the 1930s.
ADHD, its diagnosis, and its treatment have been controversial since the 1970s. For example, positions differ on whether ADHD is within the normal range of behaviour, and to degree to which ADHD is a genetic condition. Other areas of controversy include the use of stimulant medications in children, the method of diagnosis, and the possibility of overdiagnosis. In 2009, the National Institute for Health and Care Excellence states that the current treatments and methods of diagnosis are based on the dominant view of the academic literature.
Once neuroimaging studies were possible, studies in the 1990s provided support for the pre-existing theory that neurological differences (particularly in the frontal lobes) were involved in ADHD. A genetic component was identified and ADHD was acknowledged to be a persistent, long-term disorder which lasted from childhood into adulthood. ADHD was split into the current three sub-types because of a field trial completed by Lahey and colleagues and published in 1994. In 2021, global teams of scientists curated the International Consensus Statement compiling evidence-based findings about the disorder.
In 1934, Benzedrine became the first amphetamine medication approved for use in the United States. Methylphenidate was introduced in the 1950s, and enantiopure dextroamphetamine in the 1970s. The use of stimulants to treat ADHD was first described in 1937. Charles Bradley gave the children with behavioural disorders Benzedrine and found it improved academic performance and behaviour.
Research directions
Possible positive traits
Possible positive traits of ADHD are a new avenue of research, and therefore limited.
A 2020 review found that creativity may be associated with ADHD symptoms, particularly divergent thinking and quantity of creative achievements, but not with the disorder of ADHD itself – i.e. it has not been found to be increased in people diagnosed with the disorder, only in people with subclinical symptoms or those that possess traits associated with the disorder. Divergent thinking is the ability to produce creative solutions which differ significantly from each other and consider the issue from multiple perspectives. Those with ADHD symptoms could be advantaged in this form of creativity as they tend to have diffuse attention, allowing rapid switching between aspects of the task under consideration; flexible associative memory, allowing them to remember and use more distantly-related ideas which is associated with creativity; and impulsivity, allowing them to consider ideas which others may not have.
Possible biomarkers for diagnosis
Reviews of ADHD biomarkers have noted that platelet monoamine oxidase expression, urinary norepinephrine, urinary MHPG, and urinary phenethylamine levels consistently differ between ADHD individuals and non-ADHD controls. These measurements could serve as diagnostic biomarkers for ADHD, but more research is needed to establish their diagnostic utility. Urinary and blood plasma phenethylamine concentrations are lower in ADHD individuals relative to controls. The two most commonly prescribed drugs for ADHD, amphetamine and methylphenidate, increase phenethylamine biosynthesis in treatment-responsive individuals with ADHD. Lower urinary phenethylamine concentrations are associated with symptoms of inattentiveness in ADHD individuals.
See also
- Attention deficit hyperactivity disorder controversies
- Directed attention fatigue – a temporary state sharing many of the symptoms of ADHD
- Self-medication
References
- ^ Faraone SV, Bellgrove MA, Brikell I, Cortese S, Hartman CA, Hollis C, et al. (22 February 2024). "Attention-deficit/hyperactivity disorder". Nature Reviews Disease Primers. 10 (1): 11. doi:10.1038/s41572-024-00495-0. ISSN 2056-676X. PMID 38388701.
- Young K (9 February 2017). "Anxiety or ADHD? Why They Sometimes Look the Same and How to Tell the Difference". Hey Sigmund. Archived from the original on 26 January 2023. Retrieved 27 January 2023.
- ^ Institute for Health Metrics and Evaluation (17 October 2020). "Global Burden of Disease Study 2019: Attention-deficit/hyperactivity disorder—Level 3 cause" (PDF). The Lancet. 396 (10258). Table 1. Archived (PDF) from the original on 7 January 2021. Retrieved 7 January 2021.. Both DSM-IV-TR and ICD-10 criteria were used.
- ^ Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Arlington: American Psychiatric Publishing. 2013. pp. 59–65. ISBN 978-0-89042-555-8.
- ^ Diagnostic and Statistical Manual of Mental Disorders (Fifth, Text Revision (DSM-5-TR) ed.). Washington, D.C.: American Psychiatric Publishing. February 2022. ISBN 978-0-89042-575-6. OCLC 1288423302.
- ^ "6A05 Attention deficit hyperactivity disorder". International Classification of Diseases (11th ed.). February 2022 . Archived from the original on 1 August 2018. Retrieved 8 May 2022.
- ^ Foreman DM (February 2006). "Attention deficit hyperactivity disorder: legal and ethical aspects". Archives of Disease in Childhood. 91 (2): 192–194. doi:10.1136/adc.2004.064576. PMC 2082674. PMID 16428370.
- ^ Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. (September 2021). "The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder". Neuroscience and Biobehavioral Reviews. 128. Elsevier BV: 789–818. doi:10.1016/j.neubiorev.2021.01.022. ISSN 0149-7634. PMC 8328933. PMID 33549739.
- Pievsky MA, McGrath RE (March 2018). "The Neurocognitive Profile of Attention-Deficit/Hyperactivity Disorder: A Review of Meta-Analyses". Archives of Clinical Neuropsychology. 33 (2): 143–157. doi:10.1093/arclin/acx055. PMID 29106438.
- Schoechlin C, Engel RR (August 2005). "Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data". Archives of Clinical Neuropsychology. 20 (6): 727–744. doi:10.1016/j.acn.2005.04.005. PMID 15953706.
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- ^ Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, et al. (July 2019). "Brain Imaging of the Cortex in ADHD: A Coordinated Analysis of Large-Scale Clinical and Population-Based Samples". The American Journal of Psychiatry. 176 (7): 531–542. doi:10.1176/appi.ajp.2019.18091033. PMC 6879185. PMID 31014101.
- ^ Brown TE (October 2008). "ADD/ADHD and Impaired Executive Function in Clinical Practice". Current Psychiatry Reports. 10 (5): 407–411. doi:10.1007/s11920-008-0065-7. PMID 18803914. S2CID 146463279.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 148, 154–157. ISBN 978-0-07-148127-4.
DA has multiple actions in the prefrontal cortex. It promotes the 'cognitive control' of behavior: the selection and successful monitoring of behavior to facilitate attainment of chosen goals. Aspects of cognitive control in which DA plays a role include working memory, the ability to hold information 'on line' in order to guide actions, suppression of prepotent behaviors that compete with goal-directed actions, and control of attention and thus the ability to overcome distractions. Cognitive control is impaired in several disorders, including attention deficit hyperactivity disorder. ... Noradrenergic projections from the LC thus interact with dopaminergic projections from the VTA to regulate cognitive control. ... it has not been shown that 5HT makes a therapeutic contribution to treatment of ADHD.
- ^ Diamond A (2013). "Executive functions". Annual Review of Psychology. 64: 135–168. doi:10.1146/annurev-psych-113011-143750. PMC 4084861. PMID 23020641.
EFs and prefrontal cortex are the first to suffer, and suffer disproportionately, if something is not right in your life. They suffer first, and most, if you are stressed (Arnsten 1998, Liston et al. 2009, Oaten & Cheng 2005), sad (Hirt et al. 2008, von Hecker & Meiser 2005), lonely (Baumeister et al. 2002, Cacioppo & Patrick 2008, Campbell et al. 2006, Tun et al. 2012), sleep deprived (Barnes et al. 2012, Huang et al. 2007), or not physically fit (Best 2010, Chaddock et al. 2011, Hillman et al. 2008). Any of these can cause you to appear to have a disorder of EFs, such as ADHD, when you do not.
- ^ Antshel KM, Hier BO, Barkley RA (2014). "Executive Functioning Theory and ADHD". In Goldstein S, Naglieri JA (eds.). Handbook of Executive Functioning. New York, NY: Springer. pp. 107–120. doi:10.1007/978-1-4614-8106-5_7. ISBN 978-1-4614-8106-5.
- ^ Retz W, Stieglitz RD, Corbisiero S, Retz-Junginger P, Rösler M (October 2012). "Emotional dysregulation in adult ADHD: What is the empirical evidence?". Expert Review of Neurotherapeutics. 12 (10): 1241–1251. doi:10.1586/ern.12.109. PMID 23082740. S2CID 207221320.
- Faraone SV, Rostain AL, Blader J, Busch B, Childress AC, Connor DF, et al. (February 2019). "Practitioner Review: Emotional dysregulation in attention-deficit/hyperactivity disorder - implications for clinical recognition and intervention". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 60 (2): 133–150. doi:10.1111/jcpp.12899. PMID 29624671.
- Shaw P, Stringaris A, Nigg J, Leibenluft E (March 2014). "Emotion dysregulation in attention deficit hyperactivity disorder". The American Journal of Psychiatry. 171 (3): 276–293. doi:10.1176/appi.ajp.2013.13070966. PMC 4282137. PMID 24480998.
- Barkley RA (December 2002). "International Consensus Statement on ADHD". Journal of the American Academy of Child and Adolescent Psychiatry. 41 (12): 1389. doi:10.1097/00004583-200212000-00001. ISSN 0890-8567. PMID 12447019.
- Barkley RA, Murphy KR (1 June 2011). "The Nature of Executive Function (EF) Deficits in Daily Life Activities in Adults with ADHD and Their Relationship to Performance on EF Tests". Journal of Psychopathology and Behavioral Assessment. 33 (2): 137–158. doi:10.1007/s10862-011-9217-x. ISSN 1573-3505.
- Fleming M, Fitton CA, Steiner MF, McLay JS, Clark D, King A, et al. (July 2017). "Educational and Health Outcomes of Children Treated for Attention-Deficit/Hyperactivity Disorder". JAMA Pediatrics. 171 (7): e170691. doi:10.1001/jamapediatrics.2017.0691. PMC 6583483. PMID 28459927.
- Lee YC, Yang HJ, Chen VC, Lee WT, Teng MJ, Lin CH, et al. (1 April 2016). "Meta-analysis of quality of life in children and adolescents with ADHD: By both parent proxy-report and child self-report using PedsQL™". Research in Developmental Disabilities. 51–52: 160–172. doi:10.1016/j.ridd.2015.11.009. PMID 26829402.
- Barkley RA, Fischer M (July 2019). "Hyperactive Child Syndrome and Estimated Life Expectancy at Young Adult Follow-Up: The Role of ADHD Persistence and Other Potential Predictors". Journal of Attention Disorders. 23 (9): 907–923. doi:10.1177/1087054718816164. PMID 30526189. S2CID 54472439.
- Cattoi B, Alpern I, Katz JS, Keepnews D, Solanto MV (April 2022). "The Adverse Health Outcomes, Economic Burden, and Public Health Implications of Unmanaged Attention Deficit Hyperactivity Disorder (ADHD): A Call to Action Resulting from CHADD Summit, Washington, DC, October 17, 2019". Journal of Attention Disorders. 26 (6): 807–808. doi:10.1177/10870547211036754. PMID 34585995. S2CID 238218526.
- Faraone SV, Banaschewski T, Coghill D, Zheng Y, Biederman J, Bellgrove MA, et al. (1 September 2021). "The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder". Neuroscience & Biobehavioral Reviews. 128: 789–818. doi:10.1016/j.neubiorev.2021.01.022. ISSN 0149-7634. PMC 8328933. PMID 33549739.
- Barkley RA, Murphy KR (1 June 2011). "The Nature of Executive Function (EF) Deficits in Daily Life Activities in Adults with ADHD and Their Relationship to Performance on EF Tests". Journal of Psychopathology and Behavioral Assessment. 33 (2): 137–158. doi:10.1007/s10862-011-9217-x. ISSN 1573-3505.
- Groen Y, Priegnitz U, Fuermaier AB, Tucha L, Tucha O, Aschenbrenner S, et al. (December 2020). "Testing the relation between ADHD and hyperfocus experiences". Research in Developmental Disabilities. 107: 103789. doi:10.1016/j.ridd.2020.103789. PMID 33126147.
- Ayers-Glassey S, MacIntyre PD (September 2021). "Investigating emotion dysregulation and the perseveration-and flow-like characteristics of ADHD hyperfocus in Canadian undergraduate students". Psychology of Consciousness: Theory, Research, and Practice. 11 (2): 234–251. doi:10.1037/cns0000299.
- Barkley RA, Murphy KR (1 June 2011). "The Nature of Executive Function (EF) Deficits in Daily Life Activities in Adults with ADHD and Their Relationship to Performance on EF Tests". Journal of Psychopathology and Behavioral Assessment. 33 (2): 137–158. doi:10.1007/s10862-011-9217-x. ISSN 1573-3505.
- Ashinoff BK, Abu-Akel A (February 2021). "Hyperfocus: the forgotten frontier of attention". Psychological Research. 85 (1): 1–19. doi:10.1007/s00426-019-01245-8. PMC 7851038. PMID 31541305.
- Ishii S, Takagi S, Kobayashi N, Jitoku D, Sugihara G, Takahashi H (16 March 2023). "Hyperfocus symptom and internet addiction in individuals with attention-deficit/hyperactivity disorder trait". Frontiers in Psychiatry. 14: 1127777. doi:10.3389/fpsyt.2023.1127777. PMC 10061009. PMID 37009127.
- Worthington R, Wheeler S (January 2023). "Hyperfocus and offending behaviour: a systematic review" (PDF). The Journal of Forensic Practice. 25 (3): 185–200. doi:10.1108/JFP-01-2022-0005. ISSN 2050-8794. S2CID 258330884.
- Larsson H, Anckarsater H, Råstam M, Chang Z, Lichtenstein P (January 2012). "Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 53 (1): 73–80. doi:10.1111/j.1469-7610.2011.02467.x. PMID 21923806.
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. (September 2013). "Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs". Nature Genetics. 45 (9): 984–994. doi:10.1038/ng.2711. PMC 3800159. PMID 23933821.
- Cecil CA, Nigg JT (November 2022). "Epigenetics and ADHD: Reflections on Current Knowledge, Research Priorities and Translational Potential". Molecular Diagnosis & Therapy. 26 (6): 581–606. doi:10.1007/s40291-022-00609-y. PMC 7613776. PMID 35933504.
- Nigg JT, Sibley MH, Thapar A, Karalunas SL (December 2020). "Development of ADHD: Etiology, Heterogeneity, and Early Life Course". Annual Review of Developmental Psychology. 2 (1): 559–583. doi:10.1146/annurev-devpsych-060320-093413. PMC 8336725. PMID 34368774.
- ^ Barkley RA (2011). "Attention-deficit/hyperactivity disorder, self-regulation, and executive functioning.". In Vohs KD, Baumeister RF (eds.). Handbook of self-regulation: Research, theory, and applications (2nd ed.). The Guilford Press. pp. 551–563.
- Brown TE (March 2009). "ADD/ADHD and impaired executive function in clinical practice". Current Attention Disorders Reports. 1 (1): 37–41. doi:10.1007/s12618-009-0006-3. ISSN 1943-457X.
- ^ "Attention Deficit Hyperactivity Disorder (Easy-to-Read)". National Institute of Mental Health. 2013. Archived from the original on 14 April 2016. Retrieved 17 April 2016.
- Franke B, Michelini G, Asherson P, Banaschewski T, Bilbow A, Buitelaar JK, et al. (October 2018). "Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan". European Neuropsychopharmacology. 28 (10): 1059–1088. doi:10.1016/j.euroneuro.2018.08.001. PMC 6379245. PMID 30195575.
- ^ https://pure.rug.nl/ws/portalfiles/portal/1124067910/s41572-024-00495-0.pdf
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. (August 2015). "Attention-deficit/hyperactivity disorder" (PDF). Nature Reviews. Disease Primers. 1: 15020. doi:10.1038/nrdp.2015.20. PMID 27189265. S2CID 7171541.
- Meta-analysis: https://psycnet.apa.org/record/2010-02209-001
- "Intergenerational transmission of ADHD behaviors: More evidence for heritability than life history theor". europepmc.org. 2022. Retrieved 1 October 2024.
- ^ Larsson H, Chang Z, D'Onofrio BM, Lichtenstein P (July 2014). "The heritability of clinically diagnosed Attention-Deficit/Hyperactivity Disorder across the life span". Psychological Medicine. 44 (10): 2223–2229. doi:10.1017/S0033291713002493. ISSN 0033-2917. PMC 4071160. PMID 24107258.
- Sinopoli KJ, Schachar R, Dennis M (August 2011). "Traumatic brain injury and secondary attention-deficit/hyperactivity disorder in children and adolescents: the effect of reward on inhibitory control". Journal of Clinical and Experimental Neuropsychology. 33 (7): 805–819. doi:10.1080/13803395.2011.562864. PMC 3184364. PMID 21598155.
- Eme R (April 2012). "ADHD: an integration with pediatric traumatic brain injury". Expert Review of Neurotherapeutics. 12 (4): 475–483. doi:10.1586/ern.12.15. PMID 22449218. S2CID 35718630.
- Gerring JP, Brady KD, Chen A, Vasa R, Grados M, Bandeen-Roche KJ, et al. (1998). "Premorbid Prevalence of ADHD and Development of Secondary ADHD After Closed Head Injury". Journal of the American Academy of Child & Adolescent Psychiatry. 37 (6): 647–654. doi:10.1097/00004583-199806000-00015. PMID 9628085.
- Faraone SV, Larsson H (April 2019). "Genetics of attention deficit hyperactivity disorder". Molecular Psychiatry. 24 (4): 562–575. doi:10.1038/s41380-018-0070-0. PMC 6477889. PMID 29892054.
- ^ Faraone SV, Biederman J (July 2016). "Can Attention-Deficit/Hyperactivity Disorder Onset Occur in Adulthood?". JAMA Psychiatry. 73 (7): 655–656. doi:10.1001/jamapsychiatry.2016.0400. PMID 27191055.
- ^ "Facts About ADHD". Centers for Disease Control and Prevention. 6 January 2016. Archived from the original on 22 March 2016. Retrieved 20 March 2016.
- ^ "Attention-Deficit/Hyperactivity Disorder". National Institute of Mental Health. September 2023. Retrieved 2 January 2024.
- ^ "Attention-Deficit/Hyperactivity Disorder in Adults: What You Need to Know". National Institute of Mental Health. Retrieved 2 January 2024.
- Dobie C, Donald WB, Hanson M, Heim C, Huxsahl J, Karasov R, et al. (March 2012). "Diagnosis and management of attention deficit hyperactivity disorder in primary care for school-age children and adolescents". National Guideline Clearinghous. p. 79. Archived from the original on 1 March 2013. Retrieved 10 October 2012.
- ^ Ramsay JR (2007). Cognitive behavioral therapy for adult ADHD. Routledge. pp. 4, 25–26. ISBN 978-0-415-95501-0.
- Epstein JN, Loren RE (October 2013). "Changes in the Definition of ADHD in DSM-5: Subtle but Important". Neuropsychiatry. 3 (5): 455–458. doi:10.2217/npy.13.59. PMC 3955126. PMID 24644516.
- Gershon J (January 2002). "A meta-analytic review of gender differences in ADHD". Journal of Attention Disorders. 5 (3): 143–154. doi:10.1177/108705470200500302. PMID 11911007. S2CID 8076914.
- ^ Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, et al. (September 2010). "European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD". BMC Psychiatry. 10 (67): 67. doi:10.1186/1471-244X-10-67. PMC 2942810. PMID 20815868.
- Carpenter Rich E, Loo SK, Yang M, Dang J, Smalley SL (July 2009). "Social functioning difficulties in ADHD: association with PDD risk". Clinical Child Psychology and Psychiatry. 14 (3): 329–344. doi:10.1177/1359104508100890. PMC 2827258. PMID 19515751.
- Coleman WL (August 2008). "Social competence and friendship formation in adolescents with attention-deficit/hyperactivity disorder". Adolescent Medicine. 19 (2): 278–99, x. PMID 18822833.
- "ADHD Anger Management Directory". Webmd.com. Archived from the original on 5 November 2013. Retrieved 17 January 2014.
- ^ "F90 Hyperkinetic disorders". International Statistical Classification of Diseases and Related Health Problems 10th Revision. World Health Organisation. 2010. Archived from the original on 2 November 2014. Retrieved 2 November 2014.
- Bellani M, Moretti A, Perlini C, Brambilla P (December 2011). "Language disturbances in ADHD". Epidemiology and Psychiatric Sciences. 20 (4): 311–315. doi:10.1017/S2045796011000527. PMID 22201208.
- Racine MB, Majnemer A, Shevell M, Snider L (April 2008). "Handwriting performance in children with attention deficit hyperactivity disorder (ADHD)". Journal of Child Neurology. 23 (4): 399–406. doi:10.1177/0883073807309244. PMID 18401033. S2CID 206546871.
- Peterson RL, Pennington BF (May 2012). "Developmental dyslexia". Lancet. 379 (9830): 1997–2007. doi:10.1016/S0140-6736(12)60198-6. PMC 3465717. PMID 22513218.
- Sexton CC, Gelhorn HL, Bell JA, Classi PM (November 2012). "The co-occurrence of reading disorder and ADHD: epidemiology, treatment, psychosocial impact, and economic burden". Journal of Learning Disabilities. 45 (6): 538–564. doi:10.1177/0022219411407772. PMID 21757683. S2CID 385238.
- Nicolson RI, Fawcett AJ (January 2011). "Dyslexia, dysgraphia, procedural learning and the cerebellum". Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 47 (1): 117–127. doi:10.1016/j.cortex.2009.08.016. PMID 19818437. S2CID 32228208.
- "Dyslexia and ADHD". Archived from the original on 21 February 2023. Retrieved 19 May 2022.
- ^ Walitza S, Drechsler R, Ball J (August 2012). "" [The school child with ADHD]. Therapeutische Umschau (in German). 69 (8): 467–473. doi:10.1024/0040-5930/a000316. PMID 22851461.
- Frazier TW, Demaree HA, Youngstrom EA (July 2004). "Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder". Neuropsychology. 18 (3): 543–555. doi:10.1037/0894-4105.18.3.543. PMID 15291732. S2CID 17628705.
- Mackenzie GB, Wonders E (2016). "Rethinking Intelligence Quotient Exclusion Criteria Practices in the Study of Attention Deficit Hyperactivity Disorder". Frontiers in Psychology. 7: 794. doi:10.3389/fpsyg.2016.00794. PMC 4886698. PMID 27303350.
- Rommelse N, van der Kruijs M, Damhuis J, Hoek I, Smeets S, Antshel KM, et al. (December 2016). "An evidenced-based perspective on the validity of attention-deficit/hyperactivity disorder in the context of high intelligence". Neuroscience and Biobehavioral Reviews. 71: 21–47. doi:10.1016/j.neubiorev.2016.08.032. hdl:2066/163023. PMID 27590827. S2CID 6698847.
- Bridgett DJ, Walker ME (March 2006). "Intellectual functioning in adults with ADHD: a meta-analytic examination of full scale IQ differences between adults with and without ADHD". Psychological Assessment. 18 (1): 1–14. doi:10.1037/1040-3590.18.1.1. PMID 16594807.
- Young S, Hollingdale J, Absoud M, Bolton P, Branney P, Colley W, et al. (May 2020). "Guidance for identification and treatment of individuals with attention deficit/hyperactivity disorder and autism spectrum disorder based upon expert consensus". BMC Medicine. 18 (1). Springer Science and Business Media LLC: 146. doi:10.1186/s12916-020-01585-y. PMC 7247165. PMID 32448170.
- ^ "ADHD Symptoms". nhs.uk. 20 October 2017. Archived from the original on 1 February 2021. Retrieved 15 May 2018.
- ^ Bailey E (5 September 2007). "ADHD and Learning Disabilities: How can you help your child cope with ADHD and subsequent Learning Difficulties? There is a way". Remedy Health Media, LLC. Archived from the original on 3 December 2013. Retrieved 15 November 2013.
- Krull KR (5 December 2007). "Evaluation and diagnosis of attention deficit hyperactivity disorder in children". Uptodate. Wolters Kluwer Health. Archived from the original on 5 June 2009. Retrieved 12 September 2008.
- Hofvander B, Ossowski D, Lundström S, Anckarsäter H (2009). "Continuity of aggressive antisocial behavior from childhood to adulthood: The question of phenotype definition". International Journal of Law and Psychiatry. 32 (4): 224–234. doi:10.1016/j.ijlp.2009.04.004. PMID 19428109. Archived from the original on 17 May 2022. Retrieved 22 November 2021.
- Rubia K (June 2011). ""Cool" inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review". Biological Psychiatry. 69 (12). Elsevier BV/The Society of Biological Psychiatry: e69–e87. doi:10.1016/j.biopsych.2010.09.023. PMID 21094938. S2CID 14987165.
- ^ Wilens TE, Spencer TJ (September 2010). "Understanding attention-deficit/hyperactivity disorder from childhood to adulthood". Postgraduate Medicine. 122 (5): 97–109. doi:10.3810/pgm.2010.09.2206. PMC 3724232. PMID 20861593.
- Baud P, Perroud N, Aubry JM (June 2011). "". Revue Médicale Suisse (in French). 7 (297): 1219–1222. doi:10.53738/REVMED.2011.7.297.1219. PMID 21717696.
- Wilens TE, Morrison NR (July 2011). "The intersection of attention-deficit/hyperactivity disorder and substance abuse". Current Opinion in Psychiatry. 24 (4): 280–285. doi:10.1097/YCO.0b013e328345c956. PMC 3435098. PMID 21483267.
- Corkum P, Davidson F, Macpherson M (June 2011). "A framework for the assessment and treatment of sleep problems in children with attention-deficit/hyperactivity disorder". Pediatric Clinics of North America. 58 (3): 667–683. doi:10.1016/j.pcl.2011.03.004. PMID 21600348.
- Tsai MH, Huang YS (May 2010). "Attention-deficit/hyperactivity disorder and sleep disorders in children". The Medical Clinics of North America. 94 (3): 615–632. doi:10.1016/j.mcna.2010.03.008. PMID 20451036.
- Bendz LM, Scates AC (January 2010). "Melatonin treatment for insomnia in pediatric patients with attention-deficit/hyperactivity disorder". The Annals of Pharmacotherapy. 44 (1): 185–191. doi:10.1345/aph.1M365. PMID 20028959. S2CID 207263711.
- Merino-Andreu M (March 2011). "" [Attention deficit hyperactivity disorder and restless legs syndrome in children]. Revista de Neurologia (in Spanish). 52 (Suppl 1): S85–S95. doi:10.33588/rn.52S01.2011037. PMID 21365608.
- Picchietti MA, Picchietti DL (August 2010). "Advances in pediatric restless legs syndrome: Iron, genetics, diagnosis and treatment". Sleep Medicine. 11 (7): 643–651. doi:10.1016/j.sleep.2009.11.014. PMID 20620105.
- Karroum E, Konofal E, Arnulf I (2008). "". Revue Neurologique (in French). 164 (8–9): 701–721. doi:10.1016/j.neurol.2008.06.006. PMID 18656214.
- Wajszilber D, Santiseban JA, Gruber R (December 2018). "Sleep disorders in patients with ADHD: impact and management challenges". Nature and Science of Sleep. 10: 453–480. doi:10.2147/NSS.S163074. PMC 6299464. PMID 30588139.
- Long Y, Pan N, Ji S, Qin K, Chen Y, Zhang X, et al. (September 2022). "Distinct brain structural abnormalities in attention-deficit/hyperactivity disorder and substance use disorders: A comparative meta-analysis". Translational Psychiatry. 12 (1): 368. doi:10.1038/s41398-022-02130-6. PMC 9448791. PMID 36068207.
- ^ National Collaborating Centre for Mental Health (2009). "Attention Deficit Hyperactivity Disorder". Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. NICE Clinical Guidelines. Vol. 72. Leicester: British Psychological Society. pp. 18–26, 38. ISBN 978-1-85433-471-8. Archived from the original on 13 January 2016 – via NCBI Bookshelf.
- Storebø OJ, Rasmussen PD, Simonsen E (February 2016). "Association Between Insecure Attachment and ADHD: Environmental Mediating Factors" (PDF). Journal of Attention Disorders. 20 (2): 187–196. doi:10.1177/1087054713501079. PMID 24062279. S2CID 23564305. Archived (PDF) from the original on 9 December 2021. Retrieved 22 November 2021.
- Becker SP, Willcutt EG, Leopold DR, Fredrick JW, Smith ZR, Jacobson LA, et al. (June 2023). "Report of a Work Group on Sluggish Cognitive Tempo: Key Research Directions and a Consensus Change in Terminology to Cognitive Disengagement Syndrome". Journal of the American Academy of Child and Adolescent Psychiatry. 62 (6): 629–645. doi:10.1016/j.jaac.2022.07.821. PMC 9943858. PMID 36007816.
- Barkley RA (January 2014). "Sluggish cognitive tempo (concentration deficit disorder?): current status, future directions, and a plea to change the name" (PDF). Journal of Abnormal Child Psychology. 42 (1): 117–125. doi:10.1007/s10802-013-9824-y. PMID 24234590. S2CID 8287560. Archived (PDF) from the original on 9 August 2017.
- Nazar BP, Bernardes C, Peachey G, Sergeant J, Mattos P, Treasure J (December 2016). "The risk of eating disorders comorbid with attention-deficit/hyperactivity disorder: A systematic review and meta-analysis". The International Journal of Eating Disorders. 49 (12): 1045–1057. doi:10.1002/eat.22643. PMID 27859581. S2CID 38002526. Archived from the original on 8 December 2022. Retrieved 26 October 2022.
- Schneider M, VanOrmer J, Zlomke K (2019). "Adverse Childhood Experiences and Family Resilience Among Children with Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder". Journal of Developmental and Behavioral Pediatrics. 40 (8): 573–580. doi:10.1097/DBP.0000000000000703. PMID 31335581. S2CID 198193637.
- Moon DS, Bong SJ, Kim BN, Kang NR (January 2021). "Association between Maternal Adverse Childhood Experiences and Attention-Deficit/Hyperactivity Disorder in the Offspring: The Mediating Role of Antepartum Health Risks". Soa--Ch'ongsonyon Chongsin Uihak = Journal of Child & Adolescent Psychiatry. 32 (1): 28–34. doi:10.5765/jkacap.200041. PMC 7788667. PMID 33424239.
- Ford JD, Connor DF (1 June 2009). "ADHD and post-traumatic stress disorder". Current Attention Disorders Reports. 1 (2): 60–66. doi:10.1007/s12618-009-0009-0. ISSN 1943-457X. S2CID 145508751.
- Harrington KM, Miller MW, Wolf EJ, Reardon AF, Ryabchenko KA, Ofrat S (August 2012). "Attention-deficit/hyperactivity disorder comorbidity in a sample of veterans with posttraumatic stress disorder". Comprehensive Psychiatry. 53 (6): 679–690. doi:10.1016/j.comppsych.2011.12.001. PMC 6519447. PMID 22305866.
- ^ Szymanski K, Sapanski L, Conway F (1 January 2011). "Trauma and ADHD – Association or Diagnostic Confusion? A Clinical Perspective". Journal of Infant, Child, and Adolescent Psychotherapy. 10 (1). Philadelphia PA: Taylor & Francis Group: 51–59. doi:10.1080/15289168.2011.575704. eISSN 1940-9214. ISSN 1528-9168. S2CID 144348893.
- Zhang N, Gao M, Yu J, Zhang Q, Wang W, Zhou C, et al. (October 2022). "Understanding the association between adverse childhood experiences and subsequent attention deficit hyperactivity disorder: A systematic review and meta-analysis of observational studies". Brain and Behavior. 12 (10): e32748. doi:10.1002/brb3.2748. PMC 9575611. PMID 36068993.
- Nguyen MN, Watanabe-Galloway S, Hill JL, Siahpush M, Tibbits MK, Wichman C (June 2019). "Ecological model of school engagement and attention-deficit/hyperactivity disorder in school-aged children". European Child & Adolescent Psychiatry. 28 (6): 795–805. doi:10.1007/s00787-018-1248-3. PMID 30390147. S2CID 53263217.
- Miodus S, Allwood MA, Amoh N (5 January 2021). "Childhood ADHD Symptoms in Relation to Trauma Exposure and PTSD Symptoms Among College Students: Attending to and Accommodating Trauma". Journal of Emotional and Behavioral Disorders. 29 (3): 187–196. doi:10.1177/1063426620982624. ISSN 1063-4266. S2CID 234159064.
- "Is It ADHD or Trauma?". Child Mind Institute. Retrieved 18 April 2024.
- Williams AE, Giust JM, Kronenberger WG, Dunn DW (2016). "Epilepsy and attention-deficit hyperactivity disorder: links, risks, and challenges". Neuropsychiatric Disease and Treatment. 12: 287–296. doi:10.2147/NDT.S81549. PMC 4755462. PMID 26929624.
- Silva RR, Munoz DM, Alpert M (March 1996). "Carbamazepine use in children and adolescents with features of attention-deficit hyperactivity disorder: a meta-analysis". Journal of the American Academy of Child and Adolescent Psychiatry. 35 (3): 352–358. doi:10.1097/00004583-199603000-00017. PMID 8714324.
- Instanes JT, Klungsøyr K, Halmøy A, Fasmer OB, Haavik J (February 2018). "Adult ADHD and Comorbid Somatic Disease: A Systematic Literature Review". Journal of Attention Disorders (Systematic Review). 22 (3): 203–228. doi:10.1177/1087054716669589. PMC 5987989. PMID 27664125.
- Gaur S (May 2022). "The Association between ADHD and Celiac Disease in Children". Children. 9 (6). MDPI: 781. doi:10.3390/children9060781. PMC 9221618. PMID 35740718.
- Hsu TW, Chen MH, Chu CS, Tsai SJ, Bai YM, Su TP, et al. (May 2022). "Attention deficit hyperactivity disorder and risk of migraine: A nationwide longitudinal study". Headache. 62 (5): 634–641. doi:10.1111/head.14306. PMID 35524451. S2CID 248553863.
- Salem H, Vivas D, Cao F, Kazimi IF, Teixeira AL, Zeni CP (March 2018). "ADHD is associated with migraine: a systematic review and meta-analysis". European Child & Adolescent Psychiatry. 27 (3). Springer Science and Business Media LLC: 267–277. doi:10.1007/s00787-017-1045-4. PMID 28905127. S2CID 3949012.
- Pan PY, Jonsson U, Şahpazoğlu Çakmak SS, Häge A, Hohmann S, Nobel Norrman H, et al. (January 2022). "Headache in ADHD as comorbidity and a side effect of medications: a systematic review and meta-analysis". Psychological Medicine. 52 (1). Cambridge University Press: 14–25. doi:10.1017/s0033291721004141. PMC 8711104. PMID 34635194.
- Cannon Homaei S, Barone H, Kleppe R, Betari N, Reif A, Haavik J (January 2022). "ADHD symptoms in neurometabolic diseases: Underlying mechanisms and clinical implications". Neuroscience and Biobehavioral Reviews. 132: 838–856. doi:10.1016/j.neubiorev.2021.11.012. PMID 34774900. S2CID 243983688.
- Brunkhorst-Kanaan N, Libutzki B, Reif A, Larsson H, McNeill RV, Kittel-Schneider S (June 2021). "ADHD and accidents over the life span - A systematic review". Neuroscience and Biobehavioral Reviews. 125. Elsevier: 582–591. doi:10.1016/j.neubiorev.2021.02.002. PMID 33582234. S2CID 231885131.
- Vaa T (January 2014). "ADHD and relative risk of accidents in road traffic: a meta-analysis". Accident Analysis and Prevention. 62. Elsevier: 415–425. doi:10.1016/j.aap.2013.10.003. hdl:11250/2603537. PMID 24238842.
- "Attention deficit hyperactivity disorder (ADHD)". nhs.uk. 1 June 2018. Retrieved 16 February 2024.
- González-Bueso V, Santamaría JJ, Fernández D, Merino L, Montero E, Ribas J (2018). "Association between Internet Gaming Disorder or Pathological Video-Game Use and Comorbid Psychopathology: A Comprehensive Review". International Journal of Environmental Research and Public Health. 15 (4). MDPI: 668. doi:10.3390/ijerph15040668. PMC 5923710. PMID 29614059.
- Beyens I, Valkenburg PM, Piotrowski JT (2 October 2018). "Screen media use and ADHD-related behaviors: Four decades of research". PNAS USA. 115 (40). National Academy of Sciences: 9875–9881. Bibcode:2018PNAS..115.9875B. doi:10.1073/pnas.1611611114. PMC 6176582. PMID 30275318.
- de Francisco Carvalho L, Sette CP, Ferrari BL (2018). "Problematic smartphone use relationship with pathological personality traits: Systematic review and meta-analysis". Cyberpsychology: Journal of Psychosocial Research on Cyberspace. 12 (3). Masaryk University: 5. doi:10.5817/CP2018-3-5.
- Li Y, Li G, Liu L, Wu H (2020). "Correlations between mobile phone addiction and anxiety, depression, impulsivity, and poor sleep quality among college students: A systematic review and meta-analysis". Journal of Behavioral Addictions. 9 (3). Akadémiai Kiadó: 551–571. doi:10.1556/2006.2020.00057. PMC 8943681. PMID 32903205.
- Dullur P, Krishnan V, Diaz AM (2021). "A systematic review on the intersection of attention-deficit hyperactivity disorder and gaming disorder". Journal of Psychiatric Research. 133. Elsevier: 212–222. doi:10.1016/j.jpsychires.2020.12.026. PMID 33360866. S2CID 229687229.
- Gao X, Zhang M, Yang Z, Wen M, Huang H, Zheng R, et al. (2021). "Structural and Functional Brain Abnormalities in Internet Gaming Disorder and Attention-Deficit/Hyperactivity Disorder: A Comparative Meta-Analysis". Frontiers in Psychiatry. 12. Frontiers Media: 679437. doi:10.3389/fpsyt.2021.679437. PMC 8281314. PMID 34276447.
- Eirich R, McArthur BA, Anhorn C, McGuinness C, Christakis DA, Madigan S (2022). "Association of Screen Time With Internalizing and Externalizing Behavior Problems in Children 12 Years or Younger: A Systematic Review and Meta-analysis". JAMA Psychiatry. 79 (5). American Medical Association: 393–405. doi:10.1001/jamapsychiatry.2022.0155. PMC 8928099. PMID 35293954.
- Santos RM, Mendes CG, Miranda DM, Romano-Silva MA (2022). "The Association between Screen Time and Attention in Children: A Systematic Review". Developmental Neuropsychology. 47 (4). Routledge: 175–192. doi:10.1080/87565641.2022.2064863. PMID 35430923. S2CID 248228233.
- Werling AM, Kuzhippallil S, Emery S, Walitza S, Drechsler R (2022). "Problematic use of digital media in children and adolescents with a diagnosis of attention-deficit/hyperactivity disorder compared to controls. A meta-analysis". Journal of Behavioral Addictions. 11 (2). Akadémiai Kiadó: 305–325. doi:10.1556/2006.2022.00007. PMC 9295226. PMID 35567763.
- Thorell LB, Burén J, Wiman JS, Sandberg D, Nutley SB (2022). "Longitudinal associations between digital media use and ADHD symptoms in children and adolescents: a systematic literature review". European Child & Adolescent Psychiatry. 33 (8). Springer Science+Business Media: 2503–2526. doi:10.1007/s00787-022-02130-3. PMC 11272698. PMID 36562860.
- Liu H, Chen X, Huang M, Yu X, Gan Y, Wang J, et al. (2023). "Screen time and childhood attention deficit hyperactivity disorder: a meta-analysis". Reviews on Environmental Health. De Gruyter. doi:10.1515/reveh-2022-0262. PMID 37163581. S2CID 258591184.
- Augner C, Vlasak T, Barth A (2023). "The relationship between problematic internet use and attention deficit, hyperactivity and impulsivity: A meta-analysis". Journal of Psychiatric Research. 168. Elsevier: 1–12. doi:10.1016/j.jpsychires.2023.10.032. PMID 37866293. S2CID 264190691.
- Koncz P, Demetrovics Z, Takacs ZK, Griffiths MD, Nagy T, Király O (2023). "The emerging evidence on the association between symptoms of ADHD and gaming disorder: A systematic review and meta-analysis". Clinical Psychology Review. 106. Elsevier: 102343. doi:10.1016/j.cpr.2023.102343. hdl:20.500.11820/91f22260-b579-4f0b-9e81-adc063f27e9e. PMID 37883910.
- Balazs J, Kereszteny A (March 2017). "Attention-deficit/hyperactivity disorder and suicide: A systematic review". World Journal of Psychiatry. 7 (1): 44–59. doi:10.5498/wjp.v7.i1.44. PMC 5371172. PMID 28401048.
- ^ Garas P, Balazs J (21 December 2020). "Long-Term Suicide Risk of Children and Adolescents With Attention Deficit and Hyperactivity Disorder-A Systematic Review". Frontiers in Psychiatry. 11: 557909. doi:10.3389/fpsyt.2020.557909. PMC 7779592. PMID 33408650. 557909.
- ^ Septier M, Stordeur C, Zhang J, Delorme R, Cortese S (August 2019). "Association between suicidal spectrum behaviors and Attention-Deficit/Hyperactivity Disorder: A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 103: 109–118. doi:10.1016/j.neubiorev.2019.05.022. PMID 31129238. S2CID 162184004. Archived from the original on 4 November 2021. Retrieved 7 December 2021.
- Beauchaine TP, Ben-David I, Bos M (September 2020). "ADHD, financial distress, and suicide in adulthood: A population study". Science Advances. 6 (40): eaba1551. Bibcode:2020SciA....6.1551B. doi:10.1126/sciadv.aba1551. PMC 7527218. PMID 32998893. eaba1551.
- Biederman J (June 2005). "Attention-deficit/hyperactivity disorder: a selective overview". Biological Psychiatry. 57 (11): 1215–1220. doi:10.1016/j.biopsych.2004.10.020. PMID 15949990. S2CID 23671547.
- Faraone SV, Larsson H (April 2019). "Genetics of attention deficit hyperactivity disorder". Molecular Psychiatry. 24 (4): 562–575. doi:10.1038/s41380-018-0070-0. PMC 6477889. PMID 29892054. S2CID 47016805.
- Nikolas MA, Burt SA (February 2010). "Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis". Journal of Abnormal Psychology. 119 (1): 1–17. doi:10.1037/a0018010. PMID 20141238.
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, et al. (January 2019). "Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder". Nature Genetics. 51 (1): 63–75. doi:10.1038/s41588-018-0269-7. hdl:10023/20827. PMC 6481311. PMID 30478444.
- "Intergenerational transmission of ADHD behaviors: More evidence for heritability than life history theory". europepmc.org. 2022. Retrieved 12 January 2024.
- ^ Grimm O, Kranz TM, Reif A (February 2020). "Genetics of ADHD: What Should the Clinician Know?". Current Psychiatry Reports. 22 (4): 18. doi:10.1007/s11920-020-1141-x. PMC 7046577. PMID 32108282.
- Cederlöf M, Ohlsson Gotby A, Larsson H, Serlachius E, Boman M, Långström N, et al. (January 2014). "Klinefelter syndrome and risk of psychosis, autism and ADHD". Journal of Psychiatric Research. 48 (1): 128–130. doi:10.1016/j.jpsychires.2013.10.001. PMID 24139812.
- Biederman J, Spencer T (November 1999). "Attention-deficit/hyperactivity disorder (ADHD) as a noradrenergic disorder". Biological Psychiatry. 46 (9). Elsevier: 1234–1242. doi:10.1016/S0006-3223(99)00192-4. PMID 10560028. S2CID 45497168.
- Faraone SV, Larsson H (April 2019). "Genetics of attention deficit hyperactivity disorder". Molecular Psychiatry. 24 (4). Nature Research: 562–575. doi:10.1038/s41380-018-0070-0. PMC 6477889. PMID 29892054.
- Baron-Cohen S (June 2002). "The extreme male brain theory of autism". Trends in Cognitive Sciences. 6 (6). Elsevier: 248–254. doi:10.1016/S1364-6613(02)01904-6. PMID 12039606. S2CID 8098723. Archived from the original on 3 July 2013. Retrieved 9 July 2020.
- Nesse RM (2005). "32. Evolutionary Psychology and Mental Health". In Buss DM (ed.). The Handbook of Evolutionary Psychology (1st ed.). Hoboken, NJ: Wiley. p. 918. ISBN 978-0-471-26403-3.
- Nesse RM (2016) . "43. Evolutionary Psychology and Mental Health". In Buss DM (ed.). The Handbook of Evolutionary Psychology, Volume 2: Integrations (2nd ed.). Hoboken, NJ: Wiley. p. 1019. ISBN 978-1-118-75580-8.
- Esteller-Cucala P, Maceda I, Børglum AD, Demontis D, Faraone SV, Cormand B, et al. (May 2020). "Genomic analysis of the natural history of attention-deficit/hyperactivity disorder using Neanderthal and ancient Homo sapiens samples". Scientific Reports. 10 (1): 8622. Bibcode:2020NatSR..10.8622E. doi:10.1038/s41598-020-65322-4. PMC 7248073. PMID 32451437.
- Keller MC (December 2008). "The evolutionary persistence of genes that increase mental disorders risk". Current Directions in Psychological Science. 17 (6): 395–399. doi:10.1111/j.1467-8721.2008.006 (inactive 1 November 2024).
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - Barkley RA (2004). "Attention-deficit/hyperactivity disorder and self-regulation: Taking an evolutionary perspective on executive functioning.". In Baumeister RF, Vohs KD (eds.). Handbook of self-regulation: Research, theory, and applications. The Guilford Press. pp. 301–323.
- Faraone SV, Larsson H (April 2019). "Genetics of attention deficit hyperactivity disorder". Molecular Psychiatry. 24 (4). Springer Science and Business Media LLC: 562–575. doi:10.1038/s41380-018-0070-0. PMC 6477889. PMID 29892054.
- Nolen-Hoeksema S (2013). Abnormal Psychology (6th ed.). McGraw-Hill Education. p. 267. ISBN 978-0-07-803538-8.
- Skoglund C, Chen Q, D'Onofrio BM, Lichtenstein P, Larsson H (January 2014). "Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 55 (1): 61–68. doi:10.1111/jcpp.12124. PMC 4217138. PMID 25359172.
- Obel C, Zhu JL, Olsen J, Breining S, Li J, Grønborg TK, et al. (April 2016). "The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy - a re-examination using a sibling design". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 57 (4): 532–537. doi:10.1111/jcpp.12478. PMID 26511313.
- Biederman J (June 2005). "Attention-deficit/hyperactivity disorder: a selective overview". Biological Psychiatry. 57 (11): 1215–1220. doi:10.1016/j.biopsych.2004.10.020. PMID 15949990.
- Hinshaw SP (May 2018). "Attention Deficit Hyperactivity Disorder (ADHD): Controversy, Developmental Mechanisms, and Multiple Levels of Analysis". Annual Review of Clinical Psychology. 14 (1): 291–316. doi:10.1146/annurev-clinpsy-050817-084917. PMID 29220204.
- Kebir O, Joober R (December 2011). "Neuropsychological endophenotypes in attention-deficit/hyperactivity disorder: a review of genetic association studies". European Archives of Psychiatry and Clinical Neuroscience. 261 (8): 583–594. doi:10.1007/s00406-011-0207-5. PMID 21409419. S2CID 21383749.
- ^ Berry MD (January 2007). "The potential of trace amines and their receptors for treating neurological and psychiatric diseases". Reviews on Recent Clinical Trials. 2 (1): 3–19. CiteSeerX 10.1.1.329.563. doi:10.2174/157488707779318107. PMID 18473983.
Although there is little direct evidence, changes in trace amines, in particular PE, have been identified as a possible factor for the onset of attention deficit/hyperactivity disorder (ADHD). ... Further, amphetamines, which have clinical utility in ADHD, are good ligands at trace amine receptors. Of possible relevance in this aspect is modafanil, which has shown beneficial effects in ADHD patients and has been reported to enhance the activity of PE at TAAR1. Conversely, methylphenidate, ...showed poor efficacy at the TAAR1 receptor. In this respect it is worth noting that the enhancement of functioning at TAAR1 seen with modafanil was not a result of a direct interaction with TAAR1.
- Sotnikova TD, Caron MG, Gainetdinov RR (August 2009). "Trace amine-associated receptors as emerging therapeutic targets". Molecular Pharmacology. 76 (2): 229–235. doi:10.1124/mol.109.055970. PMC 2713119. PMID 19389919.
- Gizer IR, Ficks C, Waldman ID (July 2009). "Candidate gene studies of ADHD: a meta-analytic review". Human Genetics. 126 (1): 51–90. doi:10.1007/s00439-009-0694-x. PMID 19506906. S2CID 166017.
- Arcos-Burgos M, Muenke M (November 2010). "Toward a better understanding of ADHD: LPHN3 gene variants and the susceptibility to develop ADHD". Attention Deficit and Hyperactivity Disorders. 2 (3): 139–147. doi:10.1007/s12402-010-0030-2. PMC 3280610. PMID 21432600.
- Nikolaidis A, Gray JR (June 2010). "ADHD and the DRD4 exon III 7-repeat polymorphism: an international meta-analysis". Social Cognitive and Affective Neuroscience. 5 (2–3): 188–193. doi:10.1093/scan/nsp049. PMC 2894686. PMID 20019071.
- ^ Zayats T, Neale BM (12 February 2020). "Recent advances in understanding of attention deficit hyperactivity disorder (ADHD): how genetics are shaping our conceptualization of this disorder". F1000Research. 8: 2060. doi:10.12688/f1000research.18959.2. PMC 6896240. PMID 31824658.
- ^ Sonuga-Barke EJ, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. (March 2013). "Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments". The American Journal of Psychiatry. 170 (3): 275–289. doi:10.1176/appi.ajp.2012.12070991. eISSN 1535-7228. LCCN 22024537. OCLC 1480183. PMID 23360949. S2CID 434310.
Free fatty acid supplementation and artificial food color exclusions appear to have beneficial effects on ADHD symptoms, although the effect of the former are small and those of the latter may be limited to ADHD patients with food sensitivities...
- CDC (16 March 2016). "Attention-Deficit / Hyperactivity Disorder (ADHD)". Centers for Disease Control and Prevention. Archived from the original on 14 April 2016. Retrieved 17 April 2016.
- Burger PH, Goecke TW, Fasching PA, Moll G, Heinrich H, Beckmann MW, et al. (September 2011). "" [How does maternal alcohol consumption during pregnancy affect the development of attention deficit/hyperactivity syndrome in the child]. Fortschritte der Neurologie-Psychiatrie (Review) (in German). 79 (9): 500–506. doi:10.1055/s-0031-1273360. PMID 21739408. S2CID 140766296.
- Eubig PA, Aguiar A, Schantz SL (December 2010). "Lead and PCBs as risk factors for attention deficit/hyperactivity disorder". Environmental Health Perspectives (Review. Research Support, N.I.H., Extramural. Research Support, U.S. Gov't, Non-P.H.S.). 118 (12): 1654–1667. doi:10.1289/ehp.0901852. PMC 3002184. PMID 20829149.
- de Cock M, Maas YG, van de Bor M (August 2012). "Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review". Acta Paediatrica (Review. Research Support, Non-U.S. Gov't). 101 (8): 811–818. doi:10.1111/j.1651-2227.2012.02693.x. PMID 22458970. S2CID 41748237.
- Abbott LC, Winzer-Serhan UH (April 2012). "Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models". Critical Reviews in Toxicology (Review). 42 (4): 279–303. doi:10.3109/10408444.2012.658506. PMID 22394313. S2CID 38886526.
- Tiesler CM, Heinrich J (October 2014). "Prenatal nicotine exposure and child behavioural problems". European Child & Adolescent Psychiatry. 23 (10): 913–929. doi:10.1007/s00787-014-0615-y. PMC 4186967. PMID 25241028.
- Botting N, Powls A, Cooke RW, Marlow N (November 1997). "Attention deficit hyperactivity disorders and other psychiatric outcomes in very low birthweight children at 12 years". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 38 (8): 931–941. doi:10.1111/j.1469-7610.1997.tb01612.x. PMID 9413793. Archived from the original on 17 May 2022. Retrieved 22 March 2022.
- Thapar A, Cooper M, Jefferies R, Stergiakouli E (March 2012). "What causes attention deficit hyperactivity disorder?". Archives of Disease in Childhood (Review. Research Support, Non-U.S. Gov't). 97 (3): 260–265. doi:10.1136/archdischild-2011-300482. PMC 3927422. PMID 21903599.
- Millichap JG (February 2008). "Etiologic classification of attention-deficit/hyperactivity disorder". Pediatrics (Review). 121 (2): e358–e365. doi:10.1542/peds.2007-1332. PMID 18245408. S2CID 24339363.
- Eme R (April 2012). "ADHD: an integration with pediatric traumatic brain injury". Expert Review of Neurotherapeutics (Review). 12 (4): 475–483. doi:10.1586/ern.12.15. PMID 22449218. S2CID 35718630.
- ^ Mayes R, Bagwell C, Erkulwater JL (2009). Medicating Children: ADHD and Pediatric Mental Health (illustrated ed.). Harvard University Press. pp. 4–24. ISBN 978-0-674-03163-0.
- ^ Millichap JG, Yee MM (February 2012). "The diet factor in attention-deficit/hyperactivity disorder". Pediatrics. 129 (2): 330–337. doi:10.1542/peds.2011-2199. PMID 22232312. S2CID 14925322. Archived from the original on 11 September 2015.
- Tomaska LD, Brooke-Taylor S (2014). "Food Additives – General". In Motarjemi Y, Moy GG, Todd EC (eds.). Encyclopedia of Food Safety. Vol. 3 (1st ed.). Amsterdam: Elsevier/Academic Press. pp. 449–54. ISBN 978-0-12-378613-5. OCLC 865335120.
- "Background Document for the Food Advisory Committee: Certified Color Additives in Food and Possible Association with Attention Deficit Hyperactivity Disorder in Children" (PDF). U.S. Food and Drug Administration. March 2011. Archived (PDF) from the original on 6 November 2015.
- ^ Nigg JT, Holton K (October 2014). "Restriction and elimination diets in ADHD treatment". Child and Adolescent Psychiatric Clinics of North America (Review). 23 (4): 937–953. doi:10.1016/j.chc.2014.05.010. PMC 4322780. PMID 25220094.
an elimination diet produces a small aggregate effect but may have greater benefit among some children. Very few studies enable proper evaluation of the likelihood of response in children with ADHD who are not already preselected based on prior diet response.
- "Mental health of children and adolescents" (PDF). WHO Europe. 15 January 2005. Archived from the original (PDF) on 24 October 2009. Retrieved 13 October 2011.
- ^ National Collaborating Centre for Mental Health (2009). Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. NICE Clinical Guidelines. Vol. 72. Leicester: British Psychological Society. ISBN 978-1-85433-471-8. Archived from the original on 13 January 2016 – via NCBI Bookshelf.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapters 10 and 13". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 266, 315, 318–323. ISBN 978-0-07-148127-4.
Early results with structural MRI show thinning of the cerebral cortex in ADHD subjects compared with age-matched controls in prefrontal cortex and posterior parietal cortex, areas involved in working memory and attention.
- ^ Chandler DJ, Waterhouse BD, Gao WJ (May 2014). "New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons". Frontiers in Neural Circuits. 8: 53. doi:10.3389/fncir.2014.00053. PMC 4033238. PMID 24904299.
- ^ Castellanos FX, Proal E (January 2012). "Large-scale brain systems in ADHD: beyond the prefrontal-striatal model". Trends in Cognitive Sciences. 16 (1): 17–26. doi:10.1016/j.tics.2011.11.007. PMC 3272832. PMID 22169776.
Recent conceptualizations of ADHD have taken seriously the distributed nature of neuronal processing. Most of the candidate networks have focused on prefrontal-striatal-cerebellar circuits, although other posterior regions are also being proposed.
- ^ Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, et al. (October 2012). "Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies". The American Journal of Psychiatry. 169 (10): 1038–1055. doi:10.1176/appi.ajp.2012.11101521. eISSN 1535-7228. LCCN 22024537. OCLC 1480183. PMC 3879048. PMID 22983386.
- Krain AL, Castellanos FX (August 2006). "Brain development and ADHD". Clinical Psychology Review. 26 (4): 433–444. doi:10.1016/j.cpr.2006.01.005. PMID 16480802.
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LS, et al. (April 2017). "Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis". The Lancet. Psychiatry. 4 (4): 310–319. doi:10.1016/S2215-0366(17)30049-4. PMC 5933934. PMID 28219628.
- Douglas PK, Gutman B, Anderson A, Larios C, Lawrence KE, Narr K, et al. (February 2018). "Hemispheric brain asymmetry differences in youths with attention-deficit/hyperactivity disorder". NeuroImage. Clinical. 18: 744–752. doi:10.1016/j.nicl.2018.02.020. PMC 5988460. PMID 29876263.
- Damiani S, Tarchi L, Scalabrini A, Marini S, Provenzani U, Rocchetti M, et al. (April 2021). "Beneath the surface: hyper-connectivity between caudate and salience regions in ADHD fMRI at rest". European Child & Adolescent Psychiatry. 30 (4): 619–631. doi:10.1007/s00787-020-01545-0. hdl:2318/1755224. PMID 32385695. S2CID 218540328.
- ^ Tarchi L, Damiani S, Fantoni T, Pisano T, Castellini G, Politi P, et al. (December 2022). "Centrality and interhemispheric coordination are related to different clinical/behavioral factors in attention deficit/hyperactivity disorder: a resting-state fMRI study". Brain Imaging and Behavior. 16 (6): 2526–2542. doi:10.1007/s11682-022-00708-8. PMC 9712307. PMID 35859076.
- Mohamed SM, Börger NA, Geuze RH, van der Meere JJ (2015). "Brain lateralization and self-reported symptoms of ADHD in a population sample of adults: a dimensional approach". Frontiers in Psychology. 6: 1418. doi:10.3389/fpsyg.2015.01418. PMC 4585266. PMID 26441789.
- Fusar-Poli P, Rubia K, Rossi G, Sartori G, Balottin U (March 2012). "Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis". The American Journal of Psychiatry. 169 (3): 264–272. doi:10.1176/appi.ajp.2011.11060940. eISSN 1535-7228. hdl:11577/2482784. LCCN 22024537. OCLC 1480183. PMID 22294258.
- ^ Bidwell LC, McClernon FJ, Kollins SH (August 2011). "Cognitive enhancers for the treatment of ADHD". Pharmacology, Biochemistry, and Behavior. 99 (2): 262–274. doi:10.1016/j.pbb.2011.05.002. PMC 3353150. PMID 21596055.
- Cortese S (September 2012). "The neurobiology and genetics of Attention-Deficit/Hyperactivity Disorder (ADHD): what every clinician should know". European Journal of Paediatric Neurology. 16 (5): 422–433. doi:10.1016/j.ejpn.2012.01.009. PMID 22306277.
- Lesch KP, Merker S, Reif A, Novak M (June 2013). "Dances with black widow spiders: dysregulation of glutamate signalling enters centre stage in ADHD". European Neuropsychopharmacology. 23 (6): 479–491. doi:10.1016/j.euroneuro.2012.07.013. PMID 22939004. S2CID 14701654.
- Skodzik T, Holling H, Pedersen A (February 2017). "Long-Term Memory Performance in Adult ADHD". Journal of Attention Disorders. 21 (4): 267–283. doi:10.1177/1087054713510561. PMID 24232170. S2CID 27070077.
- ^ Modesto-Lowe V, Chaplin M, Soovajian V, Meyer A (July 2013). "Are motivation deficits underestimated in patients with ADHD? A review of the literature". Postgraduate Medicine. 125 (4): 47–52. doi:10.3810/pgm.2013.07.2677. PMID 23933893. S2CID 24817804.
Behavioral studies show altered processing of reinforcement and incentives in children with ADHD. These children respond more impulsively to rewards and choose small, immediate rewards over larger, delayed incentives. Interestingly, a high intensity of reinforcement is effective in improving task performance in children with ADHD. Pharmacotherapy may also improve task persistence in these children. ... Previous studies suggest that a clinical approach using interventions to improve motivational processes in patients with ADHD may improve outcomes as children with ADHD transition into adolescence and adulthood.
- B. Langguth, R. Bär, N. Wodarz, M. Wittmann, R. Laufkötter: Paradoxical reaction in ADHD. In: Deutsches Ärzteblatt international. Band 108, Nummer 31–32, August 2011, S. 541; author reply 541–541; author reply 542, (in German).doi:10.3238/arztebl.2011.0541a, PMID 21886668, PMC 3163785.
- Rainer Laufkötter, Berthold Langguth, Monika Johann, Peter Eichhammer, Göran Hajak: ADHS des Erwachsenenalters und Komorbiditäten. In: psychoneuro. 31, 2005, S. 563, (in German).doi:10.1055/s-2005-923370.
- Dulcan MK, Lake MB (2011). "Axis I Disorders Usually First Diagnosed in Infancy, Childhood or Adolescence: Attention-Deficit and Disruptive Behavior Disorders". Concise Guide to Child and Adolescent Psychiatry (4th illustrated ed.). American Psychiatric Publishing. pp. 34. ISBN 978-1-58562-416-4 – via Google Books.
- ^ Peterson BS, Trampush J, Brown M, Maglione M, Bolshakova M, Rozelle M, et al. (April 2024). "Tools for the Diagnosis of ADHD in Children and Adolescents: A Systematic Review". Pediatrics. 153 (4). doi:10.1542/peds.2024-065854. PMID 38523599.
- ^ Peterson BS, Trampush J, Maglione M, Bolshakova M, Brown M, Rozelle M, et al. (2024). "ADHD Diagnosis and Treatment in Children and Adolescents". effectivehealthcare.ahrq.gov. doi:10.23970/ahrqepccer267. PMID 38657097. Retrieved 22 June 2024.
- Smith BJ, Barkley RA, Shapiro CJ (2007). "Attention-Deficit/Hyperactivity Disorder". In Mash EJ, Barkley RA (eds.). Assessment of Childhood Disorders (4th ed.). New York, NY: Guilford Press. pp. 53–131. ISBN 978-1-59385-493-5.
- "ADHD –Pathophysiology". MerckMedicus Modules. Whitehouse Station, NJ, USA: Merck & Co., Inc. August 2002. Archived from the original on 1 May 2010.
- Al Rahbi HA, Al-Sabri RM, Chitme HR (April 2014). "Interventions by pharmacists in out-patient pharmaceutical care". Saudi Pharmaceutical Journal. 22 (2): 101–106. doi:10.1016/j.jsps.2013.04.001. PMC 3950532. PMID 24648820.
- Adamou M, Fullen T, Jones SL (25 August 2020). "EEG for Diagnosis of Adult ADHD: A Systematic Review With Narrative Analysis". Frontiers in Psychiatry. 11: 871. doi:10.3389/fpsyt.2020.00871. PMC 7477352. PMID 33192633.
- Lenartowicz A, Loo SK (November 2014). "Use of EEG to diagnose ADHD". Current Psychiatry Reports. 16 (11): 498. doi:10.1007/s11920-014-0498-0. PMC 4633088. PMID 25234074.
- ^ Singh I (December 2008). "Beyond polemics: science and ethics of ADHD". Nature Reviews. Neuroscience. 9 (12): 957–964. doi:10.1038/nrn2514. PMID 19020513. S2CID 205504587.
- Caroline SC, ed. (2010). Encyclopedia of Cross-Cultural School Psychology. Springer Science & Business Media. p. 133. ISBN 978-0-387-71798-2. Archived from the original on 22 December 2020. Retrieved 1 February 2016.
- Wiener JM, Dulcan MK (2004). Textbook Of Child and Adolescent Psychiatry (illustrated ed.). American Psychiatric Publishing. ISBN 978-1-58562-057-9. Archived from the original on 6 May 2016. Retrieved 2 November 2014.
- "Adult ADHD: Diagnosis". CAMH. Archived from the original on 21 June 2021. Retrieved 17 April 2022.
- Berger I (September 2011). "Diagnosis of attention deficit hyperactivity disorder: much ado about something" (PDF). The Israel Medical Association Journal. 13 (9): 571–574. PMID 21991721. Archived (PDF) from the original on 28 July 2020. Retrieved 23 May 2013.
- Steinau S (2013). "Diagnostic Criteria in Attention Deficit Hyperactivity Disorder - Changes in DSM 5". Frontiers in Psychiatry. 4: 49. doi:10.3389/fpsyt.2013.00049. PMC 3667245. PMID 23755024.
- Parens E, Johnston J (January 2009). "Facts, values, and attention-deficit hyperactivity disorder (ADHD): an update on the controversies". Child and Adolescent Psychiatry and Mental Health. 3 (1): 1. doi:10.1186/1753-2000-3-1. PMC 2637252. PMID 19152690.
- Szasz T (2001). "Psychiatric Medicine: Disorder". Pharmacracy: medicine and politics in America. Westport, CT: Praeger. pp. 101. ISBN 978-0-275-97196-0 – via Google Books.
Mental diseases are invented and then given a name, for example attention deficit hyperactivity disorder (ADHD).
- ^ Song P, Zha M, Yang Q, Zhang Y, Li X, Rudan I (February 2021). "The prevalence of adult attention-deficit hyperactivity disorder: A global systematic review and meta-analysis". Journal of Global Health. 11. International Global Health Society: 04009. doi:10.7189/jogh.11.04009. eISSN 2047-2986. OCLC 751737736. PMC 7916320. PMID 33692893.
- Culpepper L, Mattingly G (2010). "Challenges in identifying and managing attention-deficit/hyperactivity disorder in adults in the primary care setting: a review of the literature". Primary Care Companion to the Journal of Clinical Psychiatry. 12 (6): PCC.10r00951. doi:10.4088/PCC.10r00951pur. PMC 3067998. PMID 21494335.
- ^ Gentile JP, Atiq R, Gillig PM (August 2006). "Adult ADHD: Diagnosis, Differential Diagnosis, and Medication Management". Psychiatry. 3 (8): 25–30. PMC 2957278. PMID 20963192.
likelihood that the adult with ADHD has developed coping mechanisms to compensate for his or her impairment
- Mohr-Jensen C, Steinhausen HC (August 2016). "A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations". Clinical Psychology Review. 48: 32–42. doi:10.1016/j.cpr.2016.05.002. PMID 27390061.
- Asherson P, Agnew-Blais J (April 2019). "Annual Research Review: Does late-onset attention-deficit/hyperactivity disorder exist?". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 60 (4): 333–352. doi:10.1111/jcpp.13020. PMID 30843223.
- Consumer Reports, Drug Effectiveness Review Project (March 2012). "Evaluating Prescription Drugs Used to Treat: Attention Deficit Hyperactivity Disorder (ADHD) Comparing Effectiveness, Safety, and Price" (PDF). Best Buy Drugs: 2. Archived (PDF) from the original on 15 November 2012. Retrieved 12 April 2013.
- Owens JA (October 2008). "Sleep disorders and attention-deficit/hyperactivity disorder". Current Psychiatry Reports. 10 (5): 439–444. doi:10.1007/s11920-008-0070-x. PMID 18803919. S2CID 23624443.
- Walters AS, Silvestri R, Zucconi M, Chandrashekariah R, Konofal E (December 2008). "Review of the possible relationship and hypothetical links between attention deficit hyperactivity disorder (ADHD) and the simple sleep related movement disorders, parasomnias, hypersomnias, and circadian rhythm disorders". Journal of Clinical Sleep Medicine. 4 (6): 591–600. doi:10.5664/jcsm.27356. PMC 2603539. PMID 19110891.
- Lal C, Strange C, Bachman D (June 2012). "Neurocognitive impairment in obstructive sleep apnea". Chest. 141 (6): 1601–1610. doi:10.1378/chest.11-2214. PMID 22670023.
- Barkley RA, Benton CM (2022). Taking charge of adult ADHD: proven strategies to succeed at work, at home, and in relationships (2nd ed.). New York London: The Guilford Press. pp. 74–76. ISBN 978-1-4625-4685-5.
- Barkley RA, Benton CM (2022). "Other Mental and Emotional Problems". Taking charge of adult ADHD: proven strategies to succeed at work, at home, and in relationships (2nd ed.). New York London: The Guilford Press. ISBN 978-1-4625-4685-5.
- Peterson BS, Trampush J, Maglione M, Bolshakova M, Rozelle M, Miles J, et al. (April 2024). "Treatments for ADHD in Children and Adolescents: A Systematic Review". Pediatrics. 153 (4). doi:10.1542/peds.2024-065787. PMID 38523592.
- ^ Wigal SB (2009). "Efficacy and safety limitations of attention-deficit hyperactivity disorder pharmacotherapy in children and adults". CNS Drugs. 23 (Suppl 1): 21–31. doi:10.2165/00023210-200923000-00004. PMID 19621975. S2CID 11340058.
- ^ Mayes R, Bagwell C, Erkulwater J (2008). "ADHD and the rise in stimulant use among children". Harvard Review of Psychiatry. 16 (3): 151–166. doi:10.1080/10673220802167782. PMID 18569037. S2CID 18481191.
- ^ Coghill DR, Banaschewski T, Soutullo C, Cottingham MG, Zuddas A (November 2017). "Systematic review of quality of life and functional outcomes in randomized placebo-controlled studies of medications for attention-deficit/hyperactivity disorder". European Child & Adolescent Psychiatry. 26 (11): 1283–1307. doi:10.1007/s00787-017-0986-y. PMC 5656703. PMID 28429134.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License "CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons". Archived from the original on 16 October 2017. Retrieved 22 October 2022.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License "CC BY 4.0 Deed | Attribution 4.0 International | Creative Commons". Archived from the original on 16 October 2017. Retrieved 22 October 2022.{{cite web}}: CS1 maint: bot: original URL status unknown (link). - Jummani RR, Hirsch E, Hirsch GS (31 May 2019). "Are We Overdiagnosing and Overtreating ADHD?". Psychiatric Times. 34 (5).
- Luan R, Mu Z, Yue F, He S (2017). "Efficacy and Tolerability of Different Interventions in Children and Adolescents with Attention Deficit Hyperactivity Disorder". Frontiers in Psychiatry. 8: 229. doi:10.3389/fpsyt.2017.00229. PMC 5694170. PMID 29180967.
- ^ Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. (September 2018). "Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis". The Lancet. Psychiatry. 5 (9): 727–738. doi:10.1016/S2215-0366(18)30269-4. PMC 6109107. PMID 30097390.
- Fabiano GA, Pelham WE, Coles EK, Gnagy EM, Chronis-Tuscano A, O'Connor BC (March 2009). "A meta-analysis of behavioral treatments for attention-deficit/hyperactivity disorder". Clinical Psychology Review. 29 (2): 129–140. doi:10.1016/j.cpr.2008.11.001. PMID 19131150.
there is strong and consistent evidence that behavioral treatments are effective for treating ADHD.
- Kratochvil CJ, Vaughan BS, Barker A, Corr L, Wheeler A, Madaan V (March 2009). "Review of pediatric attention deficit/hyperactivity disorder for the general psychiatrist". The Psychiatric Clinics of North America. 32 (1): 39–56. doi:10.1016/j.psc.2008.10.001. PMID 19248915.
- Lopez PL, Torrente FM, Ciapponi A, Lischinsky AG, Cetkovich-Bakmas M, Rojas JI, et al. (March 2018). "Cognitive-behavioural interventions for attention deficit hyperactivity disorder (ADHD) in adults". The Cochrane Database of Systematic Reviews. 2018 (3): CD010840. doi:10.1002/14651858.CD010840.pub2. PMC 6494390. PMID 29566425.
- Evans SW, Owens JS, Bunford N (2014). "Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder". Journal of Clinical Child and Adolescent Psychology. 43 (4): 527–551. doi:10.1080/15374416.2013.850700. PMC 4025987. PMID 24245813.
- Van Doren J, Arns M, Heinrich H, Vollebregt MA, Strehl U, K Loo S (March 2019). "Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis". European Child & Adolescent Psychiatry. 28 (3). Springer Science and Business Media LLC: 293–305. doi:10.1007/s00787-018-1121-4. PMC 6404655. PMID 29445867.
- Enriquez-Geppert S, Smit D, Pimenta MG, Arns M (May 2019). "Neurofeedback as a Treatment Intervention in ADHD: Current Evidence and Practice". Current Psychiatry Reports. 21 (6). Springer Science and Business Media LLC: 46. doi:10.1007/s11920-019-1021-4. PMC 6538574. PMID 31139966.
- Daley D, Van Der Oord S, Ferrin M, Cortese S, Danckaerts M, Doepfner M, et al. (September 2018). "Practitioner Review: Current best practice in the use of parent training and other behavioural interventions in the treatment of children and adolescents with attention deficit hyperactivity disorder". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 59 (9). Wiley: 932–947. doi:10.1111/jcpp.12825. hdl:11343/293788. PMID 29083042. S2CID 31044370. Archived from the original on 25 September 2017. Retrieved 21 November 2018.
- Bjornstad G, Montgomery P (April 2005). Bjornstad GJ (ed.). "Family therapy for attention-deficit disorder or attention-deficit/hyperactivity disorder in children and adolescents". The Cochrane Database of Systematic Reviews (2): CD005042. doi:10.1002/14651858.CD005042.pub2. PMID 15846741. S2CID 27339381.
- Turkington C, Harris J (2009). "Attention deficit hyperactivity disorder (ADHD)". The Encyclopedia of the Brain and Brain Disorders. Infobase Publishing. pp. 47. ISBN 978-1-4381-2703-3 – via Google Books.
- Mikami AY (June 2010). "The importance of friendship for youth with attention-deficit/hyperactivity disorder". Clinical Child and Family Psychology Review. 13 (2): 181–198. doi:10.1007/s10567-010-0067-y. PMC 2921569. PMID 20490677.
- Kollins SH, DeLoss DJ, Cañadas E, Lutz J, Findling RL, Keefe RS, et al. (April 2020). "A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): a randomised controlled trial". The Lancet. Digital Health. 2 (4): e168–e178. doi:10.1016/S2589-7500(20)30017-0. PMID 33334505.
- ^ Kollins SH, Childress A, Heusser AC, Lutz J (March 2021). "Effectiveness of a digital therapeutic as adjunct to treatment with medication in pediatric ADHD". npj Digital Medicine. 4 (1): 58. doi:10.1038/s41746-021-00429-0. PMC 7997870. PMID 33772095.
- "FDA Permits Marketing of First Game-Based Digital Therapeutic to Improve Attention Function in Children with ADHD". Food and Drug Administration. United States Food and Drug Administration. 17 June 2020. Retrieved 19 April 2024.
- ^ Stamatis CA, Mercaldi C, Kollins SH (October 2023). "A Single-Arm Pivotal Trial to Assess the Efficacy of Akl-T01, a Novel Digital Intervention for Attention, in Adults Diagnosed With ADHD". Journal of the American Academy of Child & Adolescent Psychiatry. 62 (10): S318. doi:10.1016/j.jaac.2023.09.510. Retrieved 22 April 2024.
- Devilbiss DM, Berridge CW (October 2008). "Cognition-enhancing doses of methylphenidate preferentially increase prefrontal cortex neuronal responsiveness". Biological Psychiatry. 64 (7): 626–635. doi:10.1016/j.biopsych.2008.04.037. PMC 2603602. PMID 18585681.
- ^ Schulz KP, Fan J, Bédard AC, Clerkin SM, Ivanov I, Tang CY, et al. (September 2012). "Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder". Archives of General Psychiatry. 69 (9): 952–961. doi:10.1001/archgenpsychiatry.2011.2053. PMID 22945622.
- ^ Koda K, Ago Y, Cong Y, Kita Y, Takuma K, Matsuda T (July 2010). "Effects of acute and chronic administration of atomoxetine and methylphenidate on extracellular levels of noradrenaline, dopamine and serotonin in the prefrontal cortex and striatum of mice". Journal of Neurochemistry. 114 (1): 259–270. doi:10.1111/j.1471-4159.2010.06750.x. PMID 20403082.
- ^ Dodson WW (May 2005). "Pharmacotherapy of adult ADHD". Journal of Clinical Psychology. 61 (5): 589–606. doi:10.1002/jclp.20122. PMID 15723384.
For example, pseudoephedrine and ephedrine ... have no detectable effects on the symptoms of ADHD.
- Storebø OJ, Storm MR, Pereira Ribeiro J, Skoog M, Groth C, Callesen HE, et al. (March 2023). "Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD)". The Cochrane Database of Systematic Reviews. 2023 (3): CD009885. doi:10.1002/14651858.CD009885.pub3. PMC 10042435. PMID 36971690.
- ^ Ruiz-Goikoetxea M, Cortese S, Aznarez-Sanado M, Magallón S, Alvarez Zallo N, Luis EO, et al. (January 2018). "Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 84: 63–71. doi:10.1016/j.neubiorev.2017.11.007. hdl:10171/45012. PMID 29162520.
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, et al. (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". The Journal of Clinical Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- Frodl T, Skokauskas N (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects". Acta Psychiatrica Scandinavica. 125 (2): 114–126. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249. S2CID 25954331.
Basal ganglia regions like the right globus pallidus, the right putamen, and the nucleus caudatus are structurally affected in children with ADHD. These changes and alterations in limbic regions like ACC and amygdala are more pronounced in non-treated populations and seem to diminish over time from child to adulthood. Treatment seems to have positive effects on brain structure.
- Stuhec M, Lukić P, Locatelli I (February 2019). "Efficacy, Acceptability, and Tolerability of Lisdexamfetamine, Mixed Amphetamine Salts, Methylphenidate, and Modafinil in the Treatment of Attention-Deficit Hyperactivity Disorder in Adults: A Systematic Review and Meta-analysis". The Annals of Pharmacotherapy. 53 (2): 121–133. doi:10.1177/1060028018795703. PMID 30117329. S2CID 52019992.
- Faraone SV, Biederman J, Roe C (October 2002). "Comparative efficacy of Adderall and methylphenidate in attention-deficit/hyperactivity disorder: a meta-analysis". Journal of Clinical Psychopharmacology. 22 (5): 468–473. doi:10.1097/00004714-200210000-00005. PMID 12352269. S2CID 19726926.
- Nam SH, Lim MH, Park TW (April 2022). "Stimulant Induced Movement Disorders in Attention Deficit Hyperactivity Disorder". Soa--Ch'ongsonyon Chongsin Uihak = Journal of Child & Adolescent Psychiatry. 33 (2): 27–34. doi:10.5765/jkacap.210034. PMC 8984208. PMID 35418800.
- Storebø OJ, Krogh HB, Ramstad E, Moreira-Maia CR, Holmskov M, Skoog M, et al. (November 2015). "Methylphenidate for attention-deficit/hyperactivity disorder in children and adolescents: Cochrane systematic review with meta-analyses and trial sequential analyses of randomised clinical trials". BMJ. 351: h5203. doi:10.1136/bmj.h5203. PMC 4659414. PMID 26608309.
- Banaschewski T, Buitelaar J, Chui CS, Coghill D, Cortese S, Simonoff E, et al. (November 2016). "Methylphenidate for ADHD in children and adolescents: throwing the baby out with the bathwater". Evidence-Based Mental Health. 19 (4): 97–99. doi:10.1136/eb-2016-102461. PMC 10699535. PMID 27935807.
- Hoekstra PJ, Buitelaar JK (April 2016). "Is the evidence base of methylphenidate for children and adolescents with attention-deficit/hyperactivity disorder flawed?". European Child & Adolescent Psychiatry. 25 (4): 339–340. doi:10.1007/s00787-016-0845-2. PMID 27021055.
- Banaschewski T, Gerlach M, Becker K, Holtmann M, Döpfner M, Romanos M (July 2016). "Trust, but verify. The errors and misinterpretations in the Cochrane analysis by O. J. Storebo and colleagues on the efficacy and safety of methylphenidate for the treatment of children and adolescents with ADHD". Zeitschrift für Kinder- und Jugendpsychiatrie und Psychotherapie. 44 (4): 307–314. doi:10.1024/1422-4917/a000433. PMID 27270192.
- Romanos M, Reif A, Banaschewski T (September 2016). "Methylphenidate for Attention-Deficit/Hyperactivity Disorder". JAMA. 316 (9): 994–995. doi:10.1001/jama.2016.10279. PMID 27599342.
- Shaw P (May 2016). "Quantifying the Benefits and Risks of Methylphenidate as Treatment for Childhood Attention-Deficit/Hyperactivity Disorder". JAMA. 315 (18): 1953–1955. doi:10.1001/jama.2016.3427. PMID 27163984.
- Gerlach M, Banaschewski T, Coghill D, Rohde LA, Romanos M (March 2017). "What are the benefits of methylphenidate as a treatment for children and adolescents with attention-deficit/hyperactivity disorder?". Attention Deficit and Hyperactivity Disorders. 9 (1): 1–3. doi:10.1007/s12402-017-0220-2. PMID 28168407.
- Kooij JJ, Bijlenga D, Salerno L, Jaeschke R, Bitter I, Balázs J, et al. (February 2019). "Updated European Consensus Statement on diagnosis and treatment of adult ADHD". European Psychiatry. 56 (1): 14–34. doi:10.1016/j.eurpsy.2018.11.001. hdl:10651/51910. PMID 30453134.
- Boesen K, Paludan-Müller AS, Gøtzsche PC, Jørgensen KJ (February 2022). "Extended-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults". The Cochrane Database of Systematic Reviews. 2022 (2): CD012857. doi:10.1002/14651858.CD012857.pub2. PMC 8869321. PMID 35201607.
- Jaeschke RR, Sujkowska E, Sowa-Kućma M (October 2021). "Methylphenidate for attention-deficit/hyperactivity disorder in adults: a narrative review". Psychopharmacology. 238 (10): 2667–2691. doi:10.1007/s00213-021-05946-0. PMC 8455398. PMID 34436651.
- Carucci S, Balia C, Gagliano A, Lampis A, Buitelaar JK, Danckaerts M, et al. (January 2021). "Long term methylphenidate exposure and growth in children and adolescents with ADHD. A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 120: 509–525. doi:10.1016/j.neubiorev.2020.09.031. hdl:11584/301387. PMID 33080250.
- Isfandnia F, El Masri S, Radua J, Rubia K (July 2024). "The effects of chronic administration of stimulant and non-stimulant medications on executive functions in ADHD: A systematic review and meta-analysis". Neuroscience and Biobehavioral Reviews. 162 (105703): 105703. doi:10.1016/j.neubiorev.2024.105703. PMID 38718988.
- Wynchank D, Bijlenga D, Beekman AT, Kooij JJ, Penninx BW (October 2017). "Adult Attention-Deficit/Hyperactivity Disorder (ADHD) and Insomnia: an Update of the Literature". Current Psychiatry Reports. 19 (12). Springer Science and Business Media LLC: 98. doi:10.1007/s11920-017-0860-0. PMID 29086065. S2CID 38064951.
In varying percentages of trial participants, insomnia is a treatment-emergent adverse effect in triple-bead mixed amphetamine salts (40–45%), dasotraline (35–45%), lisdexamfetamine (10–19%), and extended-release methylphenidate (11%).
- Shoptaw SJ, Kao U, Ling W (January 2009). Shoptaw SJ, Ali R (eds.). "Treatment for amphetamine psychosis". The Cochrane Database of Systematic Reviews. 2009 (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMC 7004251. PMID 19160215.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Archived (PDF) from the original on 30 December 2013. Retrieved 30 December 2013.
Treatment-emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual doses. ... In a pooled analysis of multiple short-term, placebo controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
- Mosholder AD, Gelperin K, Hammad TA, Phelan K, Johann-Liang R (February 2009). "Hallucinations and other psychotic symptoms associated with the use of attention-deficit/hyperactivity disorder drugs in children". Pediatrics. 123 (2): 611–616. doi:10.1542/peds.2008-0185. PMID 19171629. S2CID 22391693.
- Ashton H, Gallagher P, Moore B (September 2006). "The adult psychiatrist's dilemma: psychostimulant use in attention deficit/hyperactivity disorder". Journal of Psychopharmacology. 20 (5): 602–610. doi:10.1177/0269881106061710. PMID 16478756. S2CID 32073083.
- Parker J, Wales G, Chalhoub N, Harpin V (September 2013). "The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials". Psychology Research and Behavior Management. 6: 87–99. doi:10.2147/PRBM.S49114. PMC 3785407. PMID 24082796.
Results suggest there is moderate-to-high-level evidence that combined pharmacological and behavioral interventions, and pharmacological interventions alone can be effective in managing the core ADHD symptoms and academic performance at 14 months. However, the effect size may decrease beyond this period. ... Only one paper examining outcomes beyond 36 months met the review criteria. ... There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
- Castells X, Blanco-Silvente L, Cunill R, et al. (Cochrane Developmental, Psychosocial and Learning Problems Group) (August 2018). "Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults". The Cochrane Database of Systematic Reviews. 2018 (8): CD007813. doi:10.1002/14651858.CD007813.pub3. PMC 6513464. PMID 30091808.
- ^ Kiely B, Adesman A (June 2015). "What we do not know about ADHD… yet". Current Opinion in Pediatrics. 27 (3): 395–404. doi:10.1097/MOP.0000000000000229. PMID 25888152. S2CID 39004402.
In addition, a consensus has not been reached on the optimal diagnostic criteria for ADHD. Moreover, the benefits and long-term effects of medical and complementary therapies for this disorder continue to be debated. These gaps in knowledge hinder the ability of clinicians to effectively recognise and treat ADHD.
- Hazell P (July 2011). "The challenges to demonstrating long-term effects of psychostimulant treatment for attention-deficit/hyperactivity disorder". Current Opinion in Psychiatry. 24 (4): 286–290. doi:10.1097/YCO.0b013e32834742db. PMID 21519262. S2CID 21998152. Archived from the original on 26 July 2020. Retrieved 19 July 2019.
- Kemper AR, Maslow GR, Hill S, Namdari B, Allen Lapointe NM, Goode AP, et al. (January 2018). "Attention Deficit Hyperactivity Disorder: Diagnosis and Treatment in Children and Adolescents". Comparative Effectiveness Reviews (203). Rockville (MD): Agency for Healthcare Research and Quality (US). PMID 29558081. Archived from the original on 17 May 2022. Retrieved 7 November 2021.
- Zhang L, Yao H, Li L, Du Rietz E, Andell P, Garcia-Argibay M, et al. (November 2022). "Risk of Cardiovascular Diseases Associated With Medications Used in Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-analysis". JAMA Network Open. 5 (11): e2243597. doi:10.1001/jamanetworkopen.2022.43597. PMC 9685490. PMID 36416824.
- Kraemer M, Uekermann J, Wiltfang J, Kis B (July 2010). "Methylphenidate-induced psychosis in adult attention-deficit/hyperactivity disorder: report of 3 new cases and review of the literature". Clinical Neuropharmacology. 33 (4): 204–206. doi:10.1097/WNF.0b013e3181e29174. PMID 20571380. S2CID 34956456.
- van de Loo-Neus GH, Rommelse N, Buitelaar JK (August 2011). "To stop or not to stop? How long should medication treatment of attention-deficit hyperactivity disorder be extended?". European Neuropsychopharmacology. 21 (8): 584–599. doi:10.1016/j.euroneuro.2011.03.008. PMID 21530185. S2CID 30068561.
- Ibrahim K, Donyai P (July 2015). "Drug Holidays From ADHD Medication: International Experience Over the Past Four Decades". Journal of Attention Disorders. 19 (7): 551–568. doi:10.1177/1087054714548035. PMID 25253684. S2CID 19949563. Archived (PDF) from the original on 30 June 2016.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 323, 368. ISBN 978-0-07-148127-4.
supervised use of stimulants at therapeutic doses may decrease risk of experimentation with drugs to self-medicate symptoms. Second, untreated ADHD may lead to school failure, peer rejection, and subsequent association with deviant peer groups that encourage drug misuse. ... amphetamines and methylphenidate are used in low doses to treat attention deficit hyperactivity disorder and in higher doses to treat narcolepsy (Chapter 12). Despite their clinical uses, these drugs are strongly reinforcing, and their long-term use at high doses is linked with potential addiction
- McDonagh MS, Christensen V, Peterson K, Thakurta S (October 2009). "Black box warnings of ADHD drugs approved by the US Food and Drug Administration". Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder: Final Report Update 3 [Internet]. Portland, Oregon: Oregon Health & Science University. Appendix G: Black box warnings of ADHD drugs approved by the US Food and Drug Administration. Archived from the original on 8 September 2017. Retrieved 17 January 2014 – via United States National Library of Medicine.
- Potter AS, Schaubhut G, Shipman M (December 2014). "Targeting the nicotinic cholinergic system to treat attention-deficit/hyperactivity disorder: rationale and progress to date". CNS Drugs. 28 (12): 1103–1113. doi:10.1007/s40263-014-0208-9. PMC 4487649. PMID 25349138.
- Perrotte G, Moreira MM, de Vargas Junior A, Teixeira Filho A, Castaldelli-Maia JM (September 2023). "Effects of Caffeine on Main Symptoms in Children with ADHD: A Systematic Review and Meta-Analysis of Randomized Trials". Brain Sciences. 13 (9): 1304. doi:10.3390/brainsci13091304. PMC 10526204. PMID 37759905.
- Turner D (April 2006). "A review of the use of modafinil for attention-deficit hyperactivity disorder". Expert Review of Neurotherapeutics. 6 (4): 455–468. doi:10.1586/14737175.6.4.455. PMID 16623645. S2CID 24293088.
- Weiss M, Tannock R, Kratochvil C, Dunn D, Velez-Borras J, Thomason C, et al. (July 2005). "A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD". Journal of the American Academy of Child and Adolescent Psychiatry. 44 (7): 647–655. doi:10.1097/01.chi.0000163280.47221.c9. PMID 15968233.
- Biederman J, Wigal SB, Spencer TJ, McGough JJ, Mays DA (February 2006). "A post hoc subgroup analysis of an 18-day randomized controlled trial comparing the tolerability and efficacy of mixed amphetamine salts extended release and atomoxetine in school-age girls with attention-deficit/hyperactivity disorder". Clinical Therapeutics. 28 (2): 280–293. doi:10.1016/j.clinthera.2006.02.008. PMID 16678649.
- Bushe C, Day K, Reed V, Karlsdotter K, Berggren L, Pitcher A, et al. (May 2016). "A network meta-analysis of atomoxetine and osmotic release oral system methylphenidate in the treatment of attention-deficit/hyperactivity disorder in adult patients". Journal of Psychopharmacology. 30 (5): 444–458. doi:10.1177/0269881116636105. PMID 27005307. S2CID 104938.
- Hazell PL, Kohn MR, Dickson R, Walton RJ, Granger RE, Wyk GW (November 2011). "Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis". Journal of Attention Disorders. 15 (8): 674–683. doi:10.1177/1087054710379737. PMID 20837981. S2CID 43503227.
- Hanwella R, Senanayake M, de Silva V (November 2011). "Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis". BMC Psychiatry. 11 (1): 176. doi:10.1186/1471-244X-11-176. PMC 3229459. PMID 22074258.
- Rezaei G, Hosseini SA, Akbari Sari A, Olyaeemanesh A, Lotfi MH, Yassini M, et al. (10 February 2016). "Comparative efficacy of methylphenidate and atomoxetine in the treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review and meta-analysis". Medical Journal of the Islamic Republic of Iran. 30: 325. PMC 4898838. PMID 27390695.
- Faraone SV, Gomeni R, Hull JT, Busse GD, Melyan Z, O'Neal W, et al. (February 2021). "Early response to SPN-812 (viloxazine extended-release) can predict efficacy outcome in pediatric subjects with ADHD: a machine learning post-hoc analysis of four randomized clinical trials". Psychiatry Research. 296: 113664. doi:10.1016/j.psychres.2020.113664. PMID 33418457. S2CID 230716405.
- Mohammadi MR, Kazemi MR, Zia E, Rezazadeh SA, Tabrizi M, Akhondzadeh S (November 2010). "Amantadine versus methylphenidate in children and adolescents with attention deficit/hyperactivity disorder: a randomized, double-blind trial". Human Psychopharmacology. 25 (7–8): 560–565. doi:10.1002/hup.1154. PMID 21312290. S2CID 30677758.
- Morrow K, Choi S, Young K, Haidar M, Boduch C, Bourgeois JA (September 2021). "Amantadine for the treatment of childhood and adolescent psychiatric symptoms". Proceedings. 34 (5): 566–570. doi:10.1080/08998280.2021.1925827. PMC 8366930. PMID 34456474.
- Stuhec M, Munda B, Svab V, Locatelli I (June 2015). "Comparative efficacy and acceptability of atomoxetine, lisdexamfetamine, bupropion and methylphenidate in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis with focus on bupropion". Journal of Affective Disorders. 178: 149–159. doi:10.1016/j.jad.2015.03.006. PMID 25813457.
- McDonagh MS, Peterson K, Thakurta S, Low A (December 2011). Drug Class Review: Pharmacologic Treatments for Attention Deficit Hyperactivity Disorder (Report). Drug Class Reviews. United States Library of Medicine. PMID 22420008. Archived from the original on 31 August 2016.
- Gurnani T, Ivanov I, Newcorn JH (February 2016). "Pharmacotherapy of Aggression in Child and Adolescent Psychiatric Disorders". Journal of Child and Adolescent Psychopharmacology. 26 (1): 65–73. doi:10.1089/cap.2015.0167. PMID 26881859.
Several studies (e.g., Findling et al. 2000; Armenteros et al. 2007) have shown that antipsychotics, especially second generation agents, can be effective when used together with stimulants for aggression in ADHD
- Childress AC, Sallee FR (March 2012). "Revisiting clonidine: an innovative add-on option for attention-deficit/hyperactivity disorder". Drugs of Today. 48 (3): 207–217. doi:10.1358/dot.2012.48.3.1750904. PMID 22462040.
- Huss M, Chen W, Ludolph AG (January 2016). "Guanfacine Extended Release: A New Pharmacological Treatment Option in Europe". Clinical Drug Investigation. 36 (1). Springer Science and Business Media LLC: 1–25. doi:10.1007/s40261-015-0336-0. PMC 4706844. PMID 26585576.
- Biederman J, Melmed RD, Patel A, McBurnett K, Konow J, Lyne A, et al. (SPD503 Study Group) (January 2008). "A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder". Pediatrics. 121 (1): e73–e84. doi:10.1542/peds.2006-3695. PMID 18166547. S2CID 25551406.
- Palumbo DR, Sallee FR, Pelham WE, Bukstein OG, Daviss WB, McDERMOTT MP (February 2008). "Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes". Journal of the American Academy of Child and Adolescent Psychiatry. 47 (2): 180–188. doi:10.1097/chi.0b013e31815d9af7. PMID 18182963.
- Arnsten AF, Jin LE (2012). "Focus: Translational Medicine: Guanfacine for the Treatment of Cognitive Disorders: A Century of Discoveries at Yale". The Yale Journal of Biology and Medicine. 85 (1): 45–58. PMC 3313539. PMID 22461743.
- ^ National Institute for Health and Care Excellence (2019). Attention deficit hyperactivity disorder: diagnosis and management. NICE Guideline, No. 87. London: National Guideline Centre (UK). ISBN 978-1-4731-2830-9. OCLC 1126668845. Archived from the original on 12 January 2021. Retrieved 9 January 2021.
- "Canadian ADHD Practice Guidelines" (PDF). Canadian ADHD Resource Alliance. Archived (PDF) from the original on 21 January 2021. Retrieved 4 February 2011.
- Stevens JR, Wilens TE, Stern TA (2013). "Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges". The Primary Care Companion for CNS Disorders. 15 (2). doi:10.4088/PCC.12f01472. PMC 3733520. PMID 23930227.
- Young JL (20 December 2010). "Individualizing Treatment for Adult ADHD: An Evidence-Based Guideline". Medscape. Archived from the original on 8 May 2022. Retrieved 8 May 2022.
- Biederman J (21 November 2003). "New-Generation Long-Acting Stimulants for the Treatment of Attention-Deficit/Hyperactivity Disorder". Medscape. Archived from the original on 8 May 2022. Retrieved 8 May 2022.
As most treatment guidelines and prescribing information for stimulant medications relate to experience in school-aged children, prescribed doses for older patients are lacking. Emerging evidence for both methylphenidate and Adderall indicate that when weight-corrected daily doses, equipotent with those used in the treatment of younger patients, are used to treat adults with ADHD, these patients show a very robust clinical response consistent with that observed in pediatric studies. These data suggest that older patients may require a more aggressive approach in terms of dosing, based on the same target dosage ranges that have already been established – for methylphenidate, 1–1.5–2 mg/kg/day, and for D,L-amphetamine, 0.5–0.75–1 mg/kg/day....
In particular, adolescents and adults are vulnerable to underdosing, and are thus at potential risk of failing to receive adequate dosage levels. As with all therapeutic agents, the efficacy and safety of stimulant medications should always guide prescribing behavior: careful dosage titration of the selected stimulant product should help to ensure that each patient with ADHD receives an adequate dose, so that the clinical benefits of therapy can be fully attained. - Kessler S (January 1996). "Drug therapy in attention-deficit hyperactivity disorder". Southern Medical Journal. 89 (1): 33–38. doi:10.1097/00007611-199601000-00005. PMID 8545689. S2CID 12798818.
- Wolraich ML, Hagan JF, Allan C, Chan E, Davison D, Earls M, et al. (October 2019). "Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents". Pediatrics. 144 (4): e20192528. doi:10.1542/peds.2019-2528. PMC 7067282. PMID 31570648.
- Ertürk E, Wouters S, Imeraj L, Lampo A (August 2020). "Association of ADHD and Celiac Disease: What Is the Evidence? A Systematic Review of the Literature". Journal of Attention Disorders (Review). 24 (10): 1371–1376. doi:10.1177/1087054715611493. PMID 26825336. S2CID 33989148.
Up till now, there is no conclusive evidence for a relationship between ADHD and CD. Therefore, it is not advised to perform routine screening of CD when assessing ADHD (and vice versa) or to implement GFD as a standard treatment in ADHD. Nevertheless, the possibility of untreated CD predisposing to ADHD-like behavior should be kept in mind. ... It is possible that in untreated patients with CD, neurologic symptoms such as chronic fatigue, inattention, pain, and headache could predispose patients to ADHD-like behavior (mainly symptoms of inattentive type), which may be alleviated after GFD treatment.
- Pelsser LM, Frankena K, Toorman J, Rodrigues Pereira R (January 2017). "Diet and ADHD, Reviewing the Evidence: A Systematic Review of Meta-Analyses of Double-Blind Placebo-Controlled Trials Evaluating the Efficacy of Diet Interventions on the Behavior of Children with ADHD". PLOS ONE (Systematic Review). 12 (1): e0169277. Bibcode:2017PLoSO..1269277P. doi:10.1371/journal.pone.0169277. PMC 5266211. PMID 28121994.
- Konikowska K, Regulska-Ilow B, Rózańska D (2012). "The influence of components of diet on the symptoms of ADHD in children". Roczniki Panstwowego Zakladu Higieny. 63 (2): 127–134. PMID 22928358.
- Arnold LE, DiSilvestro RA (August 2005). "Zinc in attention-deficit/hyperactivity disorder". Journal of Child and Adolescent Psychopharmacology. 15 (4): 619–627. doi:10.1089/cap.2005.15.619. hdl:1811/51593. PMID 16190793.
- Bloch MH, Mulqueen J (October 2014). "Nutritional supplements for the treatment of ADHD". Child and Adolescent Psychiatric Clinics of North America. 23 (4): 883–897. doi:10.1016/j.chc.2014.05.002. PMC 4170184. PMID 25220092.
- Krause J (April 2008). "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Review of Neurotherapeutics. 8 (4): 611–625. doi:10.1586/14737175.8.4.611. PMID 18416663. S2CID 24589993.
Zinc binds at ... extracellular sites of the DAT, serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc on symptoms of ADHD. It should be stated that at this time with zinc is not integrated in any ADHD treatment algorithm.
- Bálint S, Czobor P, Mészáros A, Simon V, Bitter I (2008). "" [Neuropsychological impairments in adult attention deficit hyperactivity disorder: A literature review]. Psychiatria Hungarica (in Hungarian). 23 (5). Magyar Pszichiátriai Társaság: 324–335. PMID 19129549. PsycNET 2008-18348-001.
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. (August 2015). "Attention-deficit/hyperactivity disorder". Nature Reviews. Disease Primers (Review). 1: 15020. CiteSeerX 10.1.1.497.1346. doi:10.1038/nrdp.2015.20. PMID 27189265. S2CID 7171541.
- McClernon FJ, Kollins SH (October 2008). "ADHD and smoking: from genes to brain to behavior". Annals of the New York Academy of Sciences. 1141 (1): 131–147. Bibcode:2008NYASA1141..131M. doi:10.1196/annals.1441.016. PMC 2758663. PMID 18991955.
- ^ Ginsberg Y, Quintero J, Anand E, Casillas M, Upadhyaya HP (2014). "Underdiagnosis of attention-deficit/hyperactivity disorder in adult patients: a review of the literature". The Primary Care Companion for CNS Disorders. 16 (3). doi:10.4088/PCC.13r01600. PMC 4195639. PMID 25317367.
Reports indicate that ADHD affects 2.5%–5% of adults in the general population, compared with 5%–7% of children. ... However, fewer than 20% of adults with ADHD are currently diagnosed and/or treated by psychiatrists.
- Baggio S, Fructuoso A, Guimaraes M, Fois E, Golay D, Heller P, et al. (2 August 2018). "Prevalence of Attention Deficit Hyperactivity Disorder in Detention Settings: A Systematic Review and Meta-Analysis". Frontiers in Psychiatry. 9: 331. doi:10.3389/fpsyt.2018.00331. PMC 6084240. PMID 30116206.
- "State-based Prevalence Data of Parent Reported ADHD". Centers for Disease Control and Prevention. 13 February 2017. Archived from the original on 30 March 2019. Retrieved 31 March 2020.
- ^ Willcutt EG (July 2012). "The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review". Neurotherapeutics. 9 (3): 490–499. doi:10.1007/s13311-012-0135-8. PMC 3441936. PMID 22976615.
- ^ Cowen P, Harrison P, Burns T (2012). "Drugs and other physical treatments". Shorter Oxford Textbook of Psychiatry (6th ed.). Oxford University Press. pp. 546. ISBN 978-0-19-960561-3 – via Google Books.
- Faraone SV (2011). "Ch. 25: Epidemiology of Attention Deficit Hyperactivity Disorder". In Tsuang MT, Tohen M, Jones P (eds.). Textbook of Psychiatric Epidemiology (3rd ed.). John Wiley & Sons. p. 450. ISBN 978-0-470-97740-8. Archived from the original on 22 December 2020. Retrieved 1 February 2016.
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (June 2007). "The worldwide prevalence of ADHD: a systematic review and metaregression analysis". The American Journal of Psychiatry. 164 (6): 942–948. doi:10.1176/appi.ajp.164.6.942. eISSN 1535-7228. LCCN 22024537. OCLC 1480183. PMID 17541055.
- Young S, Adamo N, Ásgeirsdóttir BB, Branney P, Beckett M, Colley W, et al. (August 2020). "Females with ADHD: An expert consensus statement taking a lifespan approach providing guidance for the identification and treatment of attention-deficit/ hyperactivity disorder in girls and women". BMC Psychiatry. 20 (1): 404. doi:10.1186/s12888-020-02707-9. PMC 7422602. PMID 32787804.
- Crawford N (February 2003). "ADHD: a women's issue". Monitor on Psychology. 34 (2): 28. Archived from the original on 9 April 2017.
- Emond V, Joyal C, Poissant H (April 2009). "" [Structural and functional neuroanatomy of attention-deficit hyperactivity disorder (ADHD)]. L'Encephale (in French). 35 (2): 107–114. doi:10.1016/j.encep.2008.01.005. PMID 19393378.
- Staller J, Faraone SV (2006). "Attention-deficit hyperactivity disorder in girls: epidemiology and management". CNS Drugs. 20 (2): 107–123. doi:10.2165/00023210-200620020-00003. PMID 16478287. S2CID 25835322.
- Schwarz A (14 December 2013). "The Selling of Attention Deficit Disorder". The New York Times. Archived from the original on 1 March 2015. Retrieved 26 February 2015.
- Whitely M, Raven M, Timimi S, Jureidini J, Phillimore J, Leo J, et al. (April 2019). "Attention deficit hyperactivity disorder late birthdate effect common in both high and low prescribing international jurisdictions: a systematic review". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 60 (4): 380–391. doi:10.1111/jcpp.12991. PMC 7379308. PMID 30317644.
- ^ Ford-Jones PC (May 2015). "Misdiagnosis of attention deficit hyperactivity disorder: 'Normal behaviour' and relative maturity". Paediatrics & Child Health. 20 (4): 200–202. doi:10.1093/pch/20.4.200. PMC 4443828. PMID 26038639.
- Connor DF (2011). "Problems of overdiagnosis and overprescribing in ADHD: are they legitimate?". Psychiatric Times. Vol. 28, no. 8. p. 14. Archived from the original on 12 August 2021.
- ^ "ADHD Throughout the Years" (PDF). Center For Disease Control and Prevention. Archived (PDF) from the original on 7 August 2013. Retrieved 2 August 2013.
- Elder TE (September 2010). "The importance of relative standards in ADHD diagnoses: evidence based on exact birth dates". Journal of Health Economics. 29 (5): 641–656. doi:10.1016/j.jhealeco.2010.06.003. PMC 2933294. PMID 20638739.
- Coker TR, Elliott MN, Toomey SL, Schwebel DC, Cuccaro P, Tortolero Emery S, et al. (September 2016). "Racial and Ethnic Disparities in ADHD Diagnosis and Treatment". Pediatrics. 138 (3): e20160407. doi:10.1542/peds.2016-0407. PMC 5684883. PMID 27553219.
There are various improvements in care that may help in closing this gap in diagnosis and treatment. These include actively and universally eliciting parental concerns about child behavior and academic performance (at home and school) at well-visits,32,33 providing care that is culturally relevant in families' preferred languages,34 and linking with community resources to provide mental health education, guidance, and services to families (eg, parent training courses for parents of children with ADHD).35–39 Pediatric clinicians also may need to consider universal behavioral health screening tools for children to improve diagnostic capabilities and recognize when a child has ADHD symptoms, even if the problem is not recognized by the parent. Because the rates of diagnosis and treatment are rising in the general population of US children, a significant need remains to identify and treat African-American and Latino children who have ADHD and avoid a widening of these disparities.
- Slobodin O, Masalha R (June 2020). "Challenges in ADHD care for ethnic minority children: A review of the current literature". Transcultural Psychiatry. 57 (3): 468–483. doi:10.1177/1363461520902885. PMID 32233772. S2CID 214768588.
- Staley BS (2024). "Attention-Deficit/Hyperactivity Disorder Diagnosis, Treatment, and Telehealth Use in Adults — National Center for Health Statistics Rapid Surveys System, United States, October–November 2023". MMWR. Morbidity and Mortality Weekly Report. 73 (40): 890–895. doi:10.15585/mmwr.mm7340a1. ISSN 0149-2195. PMC 11466376. PMID 39388378.
- Singh P (10 October 2024). "More than 15 million US adults have ADHD, new study estimates". Reuters.
- Palmer ED, Finger S (May 2001). "An early description of ADHD (inattentive subtype): Dr Alexander Crichton and 'Mental restlessness' (1798)". Child and Adolescent Mental Health. 6 (2): 66–73. doi:10.1111/1475-3588.00324.
- Crichton A (1976) . An inquiry into the nature and origin of mental derangement: comprehending a concise system of the physiology and pathology of the human mind and a history of the passions and their effects. United Kingdom: AMS Press. p. 271. ISBN 978-0-404-08212-3. Archived from the original on 3 April 2019. Retrieved 17 January 2014 – via Google Books.
- Still G (1902). "Some Abnormal Psychical Conditions in Children: The Goulstonian Lectures". Lancet. 159: 1008–1012. doi:10.1016/s0140-6736(01)74984-7.
- Millichap JG (2010). "Definition and History of ADHD". Attention Deficit Hyperactivity Disorder Handbook (2nd ed.). Springer Science. pp. 2–3. doi:10.1007/978-1-4419-1397-5_1. ISBN 978-1-4419-1396-8. LCCN 2009938108. Archived from the original on 14 January 2023. Retrieved 8 May 2022 – via Google Books.
- Weiss M, Hechtman LT, Weiss G (2001). "ADHD in Adulthood: An Introduction". ADHD in Adulthood: A Guide to Current Theory, Diagnosis, and Treatment. Taylor & Francis. pp. 34. ISBN 978-0-8018-6822-1 – via Google Books.
- Faraone SV (February 2005). "The scientific foundation for understanding attention-deficit/hyperactivity disorder as a valid psychiatric disorder". European Child & Adolescent Psychiatry. 14 (1): 1–10. doi:10.1007/s00787-005-0429-z. PMID 15756510. S2CID 143646869.
- Boseley S (30 September 2010). "Hyperactive children may have genetic disorder, says study". The Guardian. Archived from the original on 8 July 2017.
- Cormier E (October 2008). "Attention deficit/hyperactivity disorder: a review and update". Journal of Pediatric Nursing. 23 (5): 345–357. doi:10.1016/j.pedn.2008.01.003. PMID 18804015.
- National Collaborating Centre for Mental Health (2009). "Diagnosis". Attention Deficit Hyperactivity Disorder: Diagnosis and Management of ADHD in Children, Young People and Adults. NICE Clinical Guidelines. Vol. 72. Leicester: British Psychological Society. pp. 116–7, 119. ISBN 978-1-85433-471-8. Archived from the original on 13 January 2016 – via NCBI Bookshelf.
- Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT (July 1990). "Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder". Journal of the American Academy of Child and Adolescent Psychiatry. 29 (4): 526–533. doi:10.1097/00004583-199007000-00004. PMID 2387786.
- Barkley R (2006). Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. New York: Guilford. pp. 42–5. ISBN 978-1-60623-750-2. Archived from the original on 2 October 2023. Retrieved 19 July 2022.
- Lahey BB, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd GW, et al. (November 1994). "DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents". The American Journal of Psychiatry. 151 (11): 1673–1685. doi:10.1176/ajp.151.11.1673. eISSN 1535-7228. LCCN 22024537. OCLC 1480183. PMID 7943460.
- Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929-1950". Journal of the History of Medicine and Allied Sciences. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800. S2CID 24974454.
- Patrick KS, Straughn AB, Perkins JS, González MA (January 2009). "Evolution of stimulants to treat ADHD: transdermal methylphenidate". Human Psychopharmacology. 24 (1): 1–17. doi:10.1002/hup.992. PMC 2629554. PMID 19051222.
- Gross MD (February 1995). "Origin of stimulant use for treatment of attention deficit disorder". The American Journal of Psychiatry. 152 (2): 298–299. doi:10.1176/ajp.152.2.298b. eISSN 1535-7228. LCCN 22024537. OCLC 1480183. PMID 7840374.
- Brown W (1998). "Charles Bradley, M.D.". American Journal of Psychiatry. 155 (7): 968. doi:10.1176/ajp.155.7.968. eISSN 1535-7228. ISSN 0002-953X. LCCN 22024537. OCLC 1480183.
- Hoogman M, Stolte M, Baas M, Kroesbergen E (December 2020). "Creativity and ADHD: A review of behavioral studies, the effect of psychostimulants and neural underpinnings" (PDF). Neuroscience and Biobehavioral Reviews. 119: 66–85. doi:10.1016/j.neubiorev.2020.09.029. hdl:1874/409179. PMID 33035524. S2CID 222142805. Archived (PDF) from the original on 6 September 2023. Retrieved 28 August 2023.
- Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (October 2012). "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". Journal of the American Academy of Child and Adolescent Psychiatry. 51 (10): 1003–1019.e20. doi:10.1016/j.jaac.2012.08.015. PMID 23021477.
Further reading
- Barkley RA, Benton CM (2022). Taking charge of adult ADHD : proven strategies to succeed at work, at home, and in relationships (2nd ed.). The Guilford Press, a division of Guildford Publications, Inc. ISBN 9781462547524. OCLC 1251741330.
- Hallowell EM, Ratey JJ (2011). Driven to distraction : recognizing and coping with attention deficit disorder from childhood through adulthood (1 ed.). Anchor Books. ISBN 9780307743152. OCLC 1200786886.
- Hinshaw SP, Scheffler RM (2014). The ADHD Explosion: Myths, Medication, Money, and Today's Push for Performance. Oxford University Press. ISBN 978-0-19-979055-5.
- Mate G (1999). Scattered minds : a new look at the origins and healing of attention deficit disorder. Canada: Vintage. ISBN 9780676972597. OCLC 48795973.
- Schwarz A (2016). ADHD Nation: Children, Doctors, Big Pharma, and the Making of an American Epidemic. Scribner. ISBN 978-1-5011-0591-3. OCLC 951612166.
- Young JL (9 January 2007). ADHD Grown Up: A Guide to Adolescent and Adult ADHD. Norton, W. W. & Company, Inc.
- Pliszka S (July 2007). "Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder". Journal of the American Academy of Child and Adolescent Psychiatry. 46 (7): 894–921. doi:10.1097/chi.0b013e318054e724. PMID 17581453. S2CID 602465.
- Reaser A, Prevatt F, Petscher Y, Proctor B (2007). "The learning and study strategies of college students with ADHD". Psychology in the Schools. 44 (6). Wiley-Blackwell: 627–638. doi:10.1002/pits.20252. eISSN 1520-6807. ISSN 0033-3085. LCCN 64009353. OCLC 1763062.
External links
- National Institute of Mental Health. NIMH Pages About Attention-Deficit/Hyperactivity Disorder (ADHD). National Institutes of Health (NIH), U.S. Department of Health and Human Services.
 Media from Commons
Media from Commons Quotations from Wikiquote
Quotations from Wikiquote Data from Wikidata
Data from Wikidata
| Classification | D |
|---|---|
| External resources |
| Attention deficit hyperactivity disorder (ADHD) | |
|---|---|
| Main articles |
|
| Sub-types | |
| Medications |
|
| Related or outdated topics | |
| ADHD pharmacotherapies | |
|---|---|
| CNSTooltip central nervous system stimulants | |
| Non-classical CNS stimulants | |
| α2-adrenoceptor agonists | |
| Antidepressants | |
| Miscellaneous/others | |
| Related articles |
|
| Emotional and behavioral disorders | |
|---|---|
| Emotional/behavioral |
|
| Mental disorders (Classification) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| Digital media use and mental health | |
|---|---|
| Proposed or recognised diagnostic categories | |
| Disciplines involved | |
| Associated psychiatric conditions | |
| Related topics |
|