| |
| |
| Names | |
|---|---|
| Preferred IUPAC name 2,3-Dihydro-1H-indene | |
| Other names
Indan Benzocyclopentane Hydrindene 2,3-Dihydroindene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1904376 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.105 |
| Gmelin Reference | 67817 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H10 |
| Molar mass | 118.176 g/mol |
| Appearance | colorless liquid |
| Density | 0.9645 g/cm |
| Melting point | −51.4 °C (−60.5 °F; 221.8 K) |
| Boiling point | 176.5 °C (349.7 °F; 449.6 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
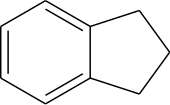
Indane or indan is an organic compound with the formula C9H10. It is a colorless liquid hydrocarbon. It is a petrochemical, a bicyclic compound. It occurs at the level of about 0.1% in coal tar. It is usually produced by hydrogenation of indene.
Derivatives
Derivatives include compounds such as 1-methylindane and 2-methylindane (where one methyl group is attached to the five carbon ring), 4-methylindane and 5-methylindane (where one methyl group is attached to the benzene ring), and various dimethylindanes. Other derivatives can be obtained indirectly, e.g. the reaction of diethyl phthalate with ethyl acetate, using metallic sodium and ethanol as a catalyst. The reaction yields indanedione ethyl ester, which can react with the sodium ions yielding a salt. This can be reversed by adding an aqueous solution of hydrochloric acid.
A family of indane derivatives are empathogen-entactogens. They are very close derivatives of other empathogen-entactogens such as MDMA and MDA. Examples include MDAI and MDMAI. Nichols also created an indane isomer of amphetamine, 2-aminoindane, NM-2-AI, and an iodized derivative 5-IAI.
See also
References
- ^ Hawley, Gessner G. (1977). The Condensed Chemical Dictionary. Van Nostrand Reinhold Company. p. 464. ISBN 0-442-23240-3.
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 602. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- Nichols, D. E; Brewster, W. K; Johnson, M. P; Oberlender, R; Riggs, R. M (1990). "Nonneurotoxic tetralin and indan analogues of 3,4-(methylenedioxy)amphetamine (MDA)". Journal of Medicinal Chemistry. 33 (2): 703–10. doi:10.1021/jm00164a037. PMID 1967651.