| This article needs more reliable medical references for verification or relies too heavily on primary sources. Please review the contents of the article and add the appropriate references if you can. Unsourced or poorly sourced material may be challenged and removed. Find sources: "Indirubin" – news · newspapers · books · scholar · JSTOR (December 2015) |  |
| |
| Names | |
|---|---|
| IUPAC name (3Z)-3-(3-Oxo-1,3-dihydro-2H-indol-2-ylidene)-1,3-dihydro-2H-indol-2-one | |
| Other names Indigo red | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.119.646 |
| EC Number |
|
| PubChem CID | |
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H10N2O2 |
| Molar mass | 262.268 g·mol |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
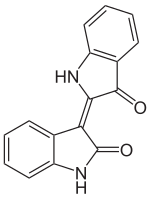
Indirubin is a chemical compound most often produced as a byproduct of bacterial metabolism. For instance, it is one of the compounds responsible for the generally benign condition purple urine bag syndrome, resulting from bacteria metabolizing indoxyl sulfate found naturally in urine.
Indirubin is a structural isomer (more precisely is position isomer) of indigo dye.
Indigo naturalis
Indirubin is a chemical constituent of indigo naturalis (also known as qing dai 青黛), which has been used since 627 AD in traditional Chinese medicine. It is essentially the indigo dye as traditionally extracted from plants by fermentation and lime treatment. The dye mixture contains a variety of organic compounds, indirubin and tryptanthrin being possible sources of some pharmacological actions. It is used in realgar/Indigo naturalis, a medication for acute promyelocytic leukemia.
Research
Indirubin exerts its effects on the human body by downregulating expression of genes. Genes PLK1 and PIN1, both oncogenic, have been shown to be affected by indirubin. Indirubin has, in vitro and in vivo, been shown to reduce expression of the CDC25B gene, which codes for production of CDC25B enzyme. CDC stands for cell-division-cycle, and is used in cellular reproduction. Studies suggest that mouse cells are viable after the CDC25B (and CDC25C) genes are "knocked out", but removal of CDC25A results in non-viable cells.
Indirubin has not been shown to prevent or treat cancer in humans. However, it is being studied for treatment of small-cell lung cancer, glioblastoma, and chronic myeloid leukemia, either alone or in conjunction with more typical cancer management treatments. It has also been studied for potential use in the treatment of ulcerative colitis, an immune-modulated disease process.
Indirubin shows anti-inflammatory and anti-angiogenesis properties in vitro.
See also
References
- ^ Qi-Yue, Y; Ting, Z; Ya-Nan, H; Sheng-Jie, H; Xuan, D; Li, H; Chun-Guang, X (14 December 2020). "From natural dye to herbal medicine: a systematic review of chemical constituents, pharmacological effects and clinical applications of indigo naturalis". Chinese Medicine. 15 (1): 127. doi:10.1186/s13020-020-00406-x. PMC 7734464. PMID 33317592.
- "Indirubin". Memorial Sloan Kettering Cancer Center. 5 May 2022.
- Williams, Shanté P.; Nowicki, Michal O.; Liu, Fang; Press, Rachael; Godlewski, Jakub; Abdel-Rasoul, Mahmoud; Kaur, Balveen; Fernandez, Soledad A.; Chiocca, E. Antonio (2011-08-15). "Indirubins Decrease Glioma Invasion by Blocking Migratory Phenotypes in Both the Tumor and Stromal Endothelial Cell Compartments". Cancer Research. 71 (16): 5374–5380. doi:10.1158/0008-5472.CAN-10-3026. ISSN 0008-5472. PMC 4288480. PMID 21697283.
- Hideo Suzuki; Tsuyoshi Kaneko; Yuji Mizokami; Toshiaki Narasaka; Shinji Endo; Hirofumi Matsui; Akinori Yanaka; Aki Hirayama; Ichinosuke Hyodo (2013). "Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis". World J Gastroenterol. 19 (17): 2718–2722. doi:10.3748/wjg.v19.i17.2718. PMC 3645393. PMID 23674882.
- Zhang, Xiaoli; Song, Yajuan; Wu, Yuanyuan; Dong, Yanmin; Lai, Li; Zhang, Jing; Lu, Binbin; Dai, Fujun; He, Lijun (2011-11-15). "Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell". International Journal of Cancer. 129 (10): 2502–2511. doi:10.1002/ijc.25909. ISSN 1097-0215. PMID 21207415. S2CID 4434671.
| Aryl hydrocarbon receptor modulators | |
|---|---|
| AhRTooltip Aryl hydrocarbon receptor |
|
| |