| |
| Names | |
|---|---|
| IUPAC name 1H-Indole-3-carbaldehyde | |
| Other names 3-Formylindole; Indole-3-carboxaldehyde; Indole-3-aldehyde | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 5-21-08-00246 |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.969 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C9H7NO |
| Molar mass | 145.161 g·mol |
| Melting point | 198 °C (388 °F; 471 K) |
| Structure | |
| Crystal structure | Orthorhombic |
| Space group | Pca21 |
| Lattice constant | a = 14.076, b = 5.8059, c = 8.6909 |
| Lattice volume (V) | 710.3 |
| Formula units (Z) | 4 |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Indole-3-carbaldehyde (I3A), also known as indole-3-aldehyde and 3-formylindole, is a metabolite of dietary L-tryptophan which is synthesized by human gastrointestinal bacteria, particularly species of the Lactobacillus genus. I3A is a biologically active metabolite which acts as a receptor agonist at the aryl hydrocarbon receptor in intestinal immune cells, in turn stimulating the production of interleukin-22 which facilitates mucosal reactivity.
Biosynthesis in humans and cellular effects
Tryptophan metabolism by human gut microbiota ()
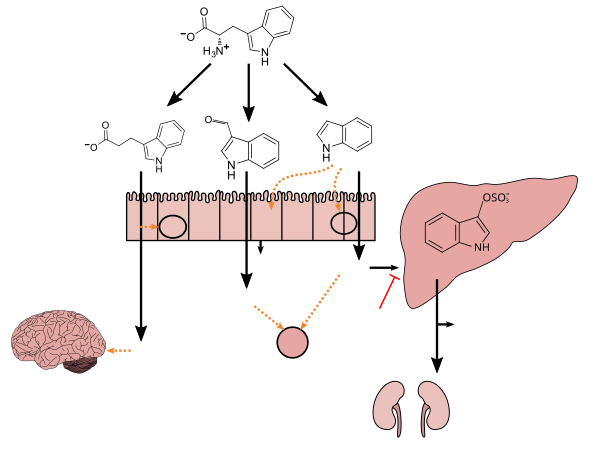 Tryptophan
Clostridium
Tryptophan
Clostridiumsporogenes Lacto- bacilli Tryptophanase- expressing bacteria IPA I3A Indole Liver Brain IPA I3A Indole Indoxyl sulfate AST-120 AhR Intestinal immune cells Intestinal epithelium PXR Mucosal homeostasis: ↓TNF-α ↑Junction protein- coding mRNAs L cell GLP-1 T J Neuroprotectant: ↓Activation of glial cells and astrocytes ↓4-Hydroxy-2-nonenal levels ↓DNA damage –Antioxidant –Inhibits β-amyloid fibril formation Maintains mucosal reactivity: ↑IL-22 production Associated with vascular disease: ↑Oxidative stress ↑Smooth muscle cell proliferation ↑Aortic wall thickness and calcification Associated with chronic kidney disease: ↑Renal dysfunction –Uremic toxin Kidneys |
Chemistry
Indole-3-carbaldehyde has reactivity typical of aromatic aldehydes. It can is easily oxidized to indole-3-carboxylic acid. It condenses with nitromethane in a Henry reaction to give 3-nitrovinyl indole.
| This section is empty. You can help by adding to it. (November 2017) |
Antifungal properties
Indole-3-carbaldehyde has antifungal properties, and partially accounts for the protection from chytridiomycosis seen in amphibian species which carry Janthinobacterium lividum on their skin.
References
- Dileep, C. S; Abdoh, M. M. M; Chakravarthy, M. P; Mohana, K. N; Sridhar, M. A (2012). "1H-Indole-3-carbaldehyde". Acta Crystallographica Section E. 68 (11): o3135. doi:10.1107/S1600536812040573. PMC 3515237. PMID 23284457.
- ^ Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. doi:10.1186/s13073-016-0296-x. PMC 4840492. PMID 27102537.
Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes . Clostridium sporogenes convert tryptophan to IPA , likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ^ "Indole-3-carboxaldehyde". PubChem Compound. United States National Library of Medicine – National Center for Biotechnology Information. 11 November 2017. Retrieved 17 November 2017.
- ROMANI LUIGINA, TERESA ZELANTE (2013). "Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22". Immunity. 39 (2): 372–385. doi:10.1016/j.immuni.2013.08.003. PMID 23973224.
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (March 2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–3703. Bibcode:2009PNAS..106.3698W. doi:10.1073/pnas.0812874106. PMC 2656143. PMID 19234110.
Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.
IPA metabolism diagram - "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 June 2018.
- Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (July 1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–21942. doi:10.1074/jbc.274.31.21937. PMID 10419516. S2CID 6630247.
has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- Brucker, Robert M.; Harris, Reid N.; Schwantes, Christian R.; Gallaher, Thomas N.; Flaherty, Devon C.; Lam, Brianna A.; Minbiole, Kevin P. C. (2008-11-01). "Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus". Journal of Chemical Ecology. 34 (11): 1422–1429. doi:10.1007/s10886-008-9555-7. ISSN 0098-0331. PMID 18949519. S2CID 9712168.