In statistical thermodynamics, thermodynamic beta, also known as coldness, is the reciprocal of the thermodynamic temperature of a system: (where T is the temperature and kB is Boltzmann constant).
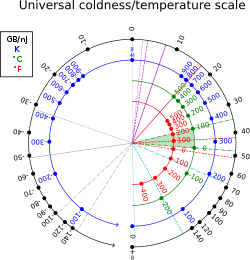
Thermodynamic beta has units reciprocal to that of energy (in SI units, reciprocal joules, ). In non-thermal units, it can also be measured in byte per joule, or more conveniently, gigabyte per nanojoule; 1 K is equivalent to about 13,062 gigabytes per nanojoule; at room temperature: T = 300K, β ≈ 44 GB/nJ ≈ 39 eV ≈ 2.4×10 J. The conversion factor is 1 GB/nJ = J.
Description
Thermodynamic beta is essentially the connection between the information theory and statistical mechanics interpretation of a physical system through its entropy and the thermodynamics associated with its energy. It expresses the response of entropy to an increase in energy. If a small amount of energy is added to the system, then β describes the amount the system will randomize.
Via the statistical definition of temperature as a function of entropy, the coldness function can be calculated in the microcanonical ensemble from the formula
(i.e., the partial derivative of the entropy S with respect to the energy E at constant volume V and particle number N).
Advantages
Though completely equivalent in conceptual content to temperature, β is generally considered a more fundamental quantity than temperature owing to the phenomenon of negative temperature, in which β is continuous as it crosses zero whereas T has a singularity.
In addition, β has the advantage of being easier to understand causally: If a small amount of heat is added to a system, β is the increase in entropy divided by the increase in heat. Temperature is difficult to interpret in the same sense, as it is not possible to "Add entropy" to a system except indirectly, by modifying other quantities such as temperature, volume, or number of particles.
Statistical interpretation
From the statistical point of view, β is a numerical quantity relating two macroscopic systems in equilibrium. The exact formulation is as follows. Consider two systems, 1 and 2, in thermal contact, with respective energies E1 and E2. We assume E1 + E2 = some constant E. The number of microstates of each system will be denoted by Ω1 and Ω2. Under our assumptions Ωi depends only on Ei. We also assume that any microstate of system 1 consistent with E1 can coexist with any microstate of system 2 consistent with E2. Thus, the number of microstates for the combined system is
We will derive β from the fundamental assumption of statistical mechanics:
- When the combined system reaches equilibrium, the number Ω is maximized.
(In other words, the system naturally seeks the maximum number of microstates.) Therefore, at equilibrium,
But E1 + E2 = E implies
So
i.e.
The above relation motivates a definition of β:
Connection of statistical view with thermodynamic view
When two systems are in equilibrium, they have the same thermodynamic temperature T. Thus intuitively, one would expect β (as defined via microstates) to be related to T in some way. This link is provided by Boltzmann's fundamental assumption written as
where kB is the Boltzmann constant, S is the classical thermodynamic entropy, and Ω is the number of microstates. So
Substituting into the definition of β from the statistical definition above gives
Comparing with thermodynamic formula
we have
where is called the fundamental temperature of the system, and has units of energy.
History
| This section's factual accuracy is disputed. Relevant discussion may be found on the talk page. Please help to ensure that disputed statements are reliably sourced. (September 2024) (Learn how and when to remove this message) |
The thermodynamic beta was originally introduced in 1971 (as Kältefunktion "coldness function") by Ingo Müller [de], one of the proponents of the rational thermodynamics school of thought, based on earlier proposals for a "reciprocal temperature" function.
See also
References
- ^ Day, W. A.; Gurtin, Morton E. (1969-01-01). "On the symmetry of the conductivity tensor and other restrictions in the nonlinear theory of heat conduction". Archive for Rational Mechanics and Analysis. 33 (1): 26–32. Bibcode:1969ArRMA..33...26D. doi:10.1007/BF00248154. ISSN 1432-0673.
- Meixner, J. (1975-09-01). "Coldness and temperature". Archive for Rational Mechanics and Analysis. 57 (3): 281–290. Bibcode:1975ArRMA..57..281M. doi:10.1007/BF00280159. ISSN 1432-0673.
- Fraundorf, P. (2003-11-01). "Heat capacity in bits". American Journal of Physics. 71 (11): 1142–1151. Bibcode:2003AmJPh..71.1142F. doi:10.1119/1.1593658. ISSN 0002-9505.
- Kittel, Charles; Kroemer, Herbert (1980), Thermal Physics (2 ed.), United States of America: W. H. Freeman and Company, ISBN 978-0471490302
- Müller, Ingo (1971). "Die Kältefunktion, eine universelle Funktion in der Thermodynamik wärmeleitender Flüssigkeiten" [The cold function, a universal function in the thermodynamics of heat-conducting liquids]. Archive for Rational Mechanics and Analysis. 40: 1–36. doi:10.1007/BF00281528.
- Müller, Ingo (1971). "The Coldness, a Universal Function in Thermoelastic Bodies". Archive for Rational Mechanics and Analysis. 41 (5): 319–332. Bibcode:1971ArRMA..41..319M. doi:10.1007/BF00281870.
- Castle, J.; Emmenish, W.; Henkes, R.; Miller, R.; Rayne, J. (1965). Science by Degrees: Temperature from Zero to Zero. New York: Walker and Company.
 (where T is the temperature and kB is
(where T is the temperature and kB is  ). In non-thermal units, it can also be measured in
). In non-thermal units, it can also be measured in  J.
J.












 is called the fundamental temperature of the system, and has units of energy.
is called the fundamental temperature of the system, and has units of energy.