| |
| Names | |
|---|---|
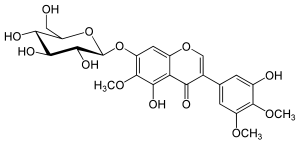
| IUPAC name 7-(β-D-Glucopyranosyloxy)-3′,5-dihydroxy-4′,5′,6-trimethoxyisoflavone | |
| Systematic IUPAC name 5-Hydroxy-3-(3-hydroxy-4,5-dimethoxyphenyl)-6-methoxy-7-{oxy}-4H-1-benzopyran-4-one | |
| Other names Irisin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C24H26O13 |
| Molar mass | 522.45 g/mol |
| Melting point | 208 °C (406 °F; 481 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Iridin is an isoflavone, a type of flavonoid. It is the 7-glucoside of irigenin and can be isolated from several species of irises like orris root, Iris florentina or Iris versicolor, also commonly known as the larger blue flag. It can also be found in Iris kemaonensis.
The compound is toxic and these plants have been mentioned as causing poisoning in humans and animals.
References
- Iridin on chestofbooks.com
- Iridin on drugs.com
- Agarwal, V.K.; Thappa, R.K.; Agarwal, S.G.; Mehraa, M.S.; Dhar, K.L. (1984). "Isoflavones of two Iris species". Phytochemistry. 23 (11): 2703–2704. Bibcode:1984PChem..23.2703A. doi:10.1016/S0031-9422(00)84141-2.
- J. B. Harborne The Flavonoids: Advances in Research since 1980, p. 133, at Google Books
- Yellow Iris on cbif.gc.ca Archived 2011-06-10 at the Wayback Machine
| Isoflavones and their glycosides | |
|---|---|
| Isoflavones | |
| O-methylated isoflavones | |
| Glycosides | |
| Prenylated isoflavones | |
| Pyranoisoflavones | |
| Derivatives | |
| Synthetic | |
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |