| |||
| Names | |||
|---|---|---|---|
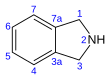
| Preferred IUPAC name 2,3-Dihydro-1H-isoindole | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.156.955 | ||
| PubChem CID | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C8H9N | ||
| Molar mass | 119.167 g·mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. lenalidomide and pazinaclone.

Substituted isoindolines
1-Substituted isoindolines and isoindolinones are chiral. Isoindolylcarboxylic acid and 1,3-disubstituted isoindolines are constituents of some pharmaceuticals and natural products. Isoindolines can be prepared by 1,2-addition of a nucleophile onto a bifunctional ε-benzoiminoenoates followed by intramolecular aza-Michael reaction. Another route involves cycloaddition of the azomethine ylides (e.g. (CH2)2NR) to quinone in the presence of suitable catalysts. These methods have also been adapted to give chiral derivatives.
Related compounds
- 4,7-Dihydroisoindole
- Indole
- Indene
- Indoline
- Benzofuran
- Carbazole
- Carboline
- Isatin
- Methylindole
- Oxindole
- Pyrrole
- Skatole
- Benzene
References
- Isoindoline
- Isoindoline
- Speck Klaus; Magauer Thomas "The chemistry of isoindole natural products" Beilstein journal of organic chemistry 2013, vol. 9, pp. 2048-78. doi:10.3762/bjoc.9.243
- Pandey, G.; Varkhedkar, R.; Tiwari, D (2015) Efficient Access to Enantiopure 1,3-disubstituted Isoindolines from Selective Catalytic Fragmentation of Original Desymmetrized Rigid Overbred Template, Org. Biomol. Chem., DOI: 10.1039/C5OB00229J
- A Facile Access to Enantioenriched Isoindolines via One-Pot Sequential Cu(I)-Catalyzed Asymmetric 1,3-Dipolar Cycloaddition/Aromatization DOI: 10.1021/ol302987h
- Asymmetric organocatalytic formal double-arylation of azomethines for the synthesis of highly enantiomerically enriched isoindolines DOI: 10.1039/B917246G