| |
| Names | |
|---|---|
| Other names 1,3-Benzenedialdehyde, 1,3-Diformylbenzene | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.942 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H6O2 |
| Molar mass | 134.134 g·mol |
| Appearance | white |
| Density | 1.395 g/cm |
| Melting point | 89.5 °C (193.1 °F; 362.6 K) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
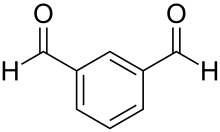
Isophthalaldehyde is an organic compound with the formula C6H4(CHO)2. It is one of three isomers of benzene dicarbaldehyde, a reduced analog of phthalic acid. It is a colorless solid, although commercial samples often appear yellowish. One preparation entails the Sommelet reaction of α,α'-diamino-ortho-xylene.
Reactions and use
Like many benzaldehydes, isophthalaldehyde forms a variety of Schiff base derivatives. Being bifunctional (having two formyl groups), isophthalaldehyde allows the formation of polymers or covalent organic frameworks upon reaction with di- or triamines. They also find use in metal coordination complexes.
Related compounds
References
- Britton, Doyle (2002). "O- andm-Benzenedicarbaldehyde". Acta Crystallographica Section C Crystal Structure Communications. 58 (11): o637–o639. doi:10.1107/S0108270102015597. PMID 12415166. S2CID 7685455.
- "Isophthalaldehyde". pubchem.ncbi.nlm.nih.gov. Retrieved 3 February 2022.
- Ackerman, J. H.; Surrey, A. R. (1967). "Isophthalaldehyde". Organic Syntheses. 47: 76. doi:10.15227/orgsyn.047.0076.
- Schwab, Matthias Georg; Fassbender, Birgit; Spiess, Hans Wolfgang; Thomas, Arne; Feng, Xinliang; Müllen, Klaus (2009). "Catalyst-free Preparation of Melamine-Based Microporous Polymer Networks through Schiff Base Chemistry". J. Am. Chem. Soc. 131 (21): 7216–7217. doi:10.1021/ja902116f. PMID 19469570.
- Schoustra, Sybren K.; Smulders, Maarten M. J. (2023). "Metal Coordination in Polyimine Covalent Adaptable Networks for Tunable Material Properties and Enhanced Creep Resistance". Macromolecular Rapid Communications. 44 (5): 2200790. doi:10.1002/marc.202200790. PMID 36629864. S2CID 255593988.
- Nasr, G.; Macron, T.; Gilles, A.; Mouline, Z.; Barboiu, M. (2012). "Metallodynameric membranes – toward the constitutional transport of gases". Chemical Communications. 48 (54): 6827–6829. doi:10.1039/C2CC32656F.