| Japanese tree frog | |
|---|---|

| |
| Hyla japonica resting on a plant. | |
| Conservation status | |
 Least Concern (IUCN 3.1) | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Anura |
| Family: | Hylidae |
| Genus: | Hyla |
| Species: | H. japonica |
| Binomial name | |
| Hyla japonica (Günther, 1859) | |
| Synonyms | |
| |
Hyla japonica, commonly known as the Japanese tree frog, is a species of anuran native to Japan, China, and Korea. H. japonica is unique in its ability to withstand extreme cold, with some individuals showing cold resistance at temperatures as low as −30 °C for up to 120 days. H. japonica are not currently facing any notable risk of extinction and are classified by the IUCN as a species of "least concern". Notably, H. japonica have been sent to space in a study that explored the effect of microgravity on H. japonica. Hyla japonica is synonymous with Dryophytes japonicus.
The Japanese tree frog lives in a variety of habitats such as wetlands, forests, rivers, and mountains. They are generally located near vegetation near water sources and forests. They are carnivores that prey on insects and spiders. Their average litter size is around 340–1,500 eggs, and their lifespan is usually around six years. There is an estimated 100 million of these frogs in Japan, but the accuracy is limited due to difficulty in counting.
Taxonomy
Some authorities use the scientific name, Hyla japonica, in reference to the Japanese tree frog. The binomial name, Dryophytes japonicus, is also sometimes used. Studies have characterized the relationship between H. suweonensis and H. japonica. H. suweonensis is a closely related species to H. japonica. In general, H. suweonensis is smaller and more slender than H. japonica. The distance between nostril and upper lip (NL), distance between posterior corners of eyes (EPD), distance between semi-minor axis of the upper eye (LILe), angle between the two lines that connect the posterior corner of the eyes and ipsilateral nostrils (αEPD-N), and the angle between the two lines that connect the anterior corner of the eyes and the ipsilateral nostrils (αEAD-N) can all be used to differentiate between H. suweonensis and H. japonica.
Description
H. japonica are on average 32.81±0.96 mm in length. They have an average skull width of 12.02±0.36 mm and an average skull length of 9.38±0.14 mm. The dorsal body of H. japonica is green/brown and the ventral body is white. H. japonica is also characterized by a dark spot on the upper lip below the eye. Female H. japonica, on average, are larger in size compared to male H. japonica. H. japonica has a dark vocal sac.
Abnormal coloration
Some H. japonica are abnormally colored. Frogs observed in South Korea were found to be entirely blue, while others yellow, with green dorsal patterns. Another frog found in Russia was observed to be fully blue, and was captured for observation, where it ultimately returned to a green/brown color. Specific reasons behind such observations in color are currently unexplained, but mutations and maladaptations have been put forth by scientists as possible explanations. Further work must be conducted in order to elucidate the mechanisms behind these color changes.
Habitat and distribution
H. japonica are found in many parts of Asia, specifically in Japan, China, Korea, Mongolia, and Russia. H. japonica inhabits forest-like environments, bushlands, meadows, swamps, and river valleys. H. japonica, like most frog species, inhabit locations with both aquatic and terrestrial features. This is due to the necessity of the frog life cycle for both water and land.
Changes in availability of native H. japonica habitats have resulted in rice paddies serving as lodging for H. japonica. H. japonica seems to be able to inhabit these rice paddies successfully and have a demonstrated preference for sites high in vegetation.

Behavior
The behavior of H. japonica when exposed to microgravity has been experimentally investigated. These frogs, under such microgravity conditions, would bend their neck backwards. These frogs would also walk backwards, an observation consistent with the behavior of sick frogs. The combination of neck backwards movement and backward walking could be indicators of motion sickness in the frogs. H. japonica were shown to adapt to the microgravity and were able to improve their jumping and perching activity over time. H. japonica, under micro-gravitational conditions, were also observed to attempt to eat but were unable to ingest the food. All the frogs that were sent to space were safely recovered and were observed to resume normal function after 2.5 hours back under normal gravity.
Diet
Hyla japonica forages in both breeding and non-breeding seasons. H. japonica are known to be opportunistic predators. This feature of H. japonica was discovered through analysis that showed a strong correlation between the relative abundance of organisms in a given environment and the prey composition H. japonica for that environment. A highest percentage of H. japonica's diet is ants, followed by beetles and caterpillars. There does not appear to be a significant difference in the diet composition between the two sexes of H. japonica. However, during the breeding season, males have a higher chance of having an empty stomach due to the heightened energetic cost imposed by breeding.
Mating
Mating system
Male H. japonica are observed to congregate in leks in an attempt to mate with female H. japonica. A lek is an area where males will congregate in order to perform courtship displays in order to mate with females. Male leks seem to form preferentially at spots with significant water resources. Female distribution appears to be skewed towards male lekking sites. These lekking sites were identified by their extremely high male density. Female distribution does not seem to be explained by other factors like water availability, vegetation, or herbicide levels. The lek model that seems to fit the lekking exhibited by H. japonica is the environmental hotspot model. This is because the sites that had the highest male density were those that had significantly high female encounter rates. Thus, there seems to be some bias of lek location towards areas with high female densities. Females need water for oviposition and the preference of male leks to form near water could be a mediating factor in choosing spots close to females.
Calling
Male H. japonica will call to signal their presence to females and to compete with other males. Notes of H. japonica calls are made up of fine pulses, and exist mainly at the frequency of 1.7 kHz. H. japonica was observed to make the majority, if not all, of their calls at night. H. japonica also seemed to call when they were located on the banks of rice paddies. Note length and note interval were observed to decrease in H. japonica males when temperature increased.
Preference
H. japonica are observed to prefer more shallow and smaller bodies of water for breeding. H. japonica prefer bodies of water termed oxbow lakes, likely due to their freestanding nature and higher chance of being refilled. Oxbow lakes are likely preferred due to the inability of tadpoles to swim along or against strong currents.
Infection effects on calling
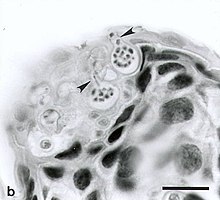
H. japonica is susceptible to infection by Batrachochytrium dendrobatidis. Batrachochytrium dendrobatidis infection causes a disease termed Chytridiomycosis. Chytridiomycosis is an amphibian disease that has devastated many amphibian populations across the world. H. japonica seems to be susceptible to Chytridiomycosis, however the disease does not appear to pose a high burden to this species. In fact, H. japonica has not been observed to suffer from increased morbidity or mortality from Chytridiomycosis H. japonica in Korea seem to have Batrachochytrium dendrobatidis infection rates ranging from 10.6 to16.2%.
Chytridiomycosis has been observed to affect the calling of H. japonica in a multitude of different ways. Number of pulses per note and note duration were both observed to be significantly higher in infected H. japonica compared to uninfected H. japonica.
The increased effort devoted to reproductive efforts by infected H. japonica is an interesting result that warrants further research. Two hypotheses have been proposed to explain the observed behavior. First, this increased investment towards reproduction might be a result of Batrachochytrium dendrobatidis driving increased reproduction in order to increase spread of infection. Another hypothesis is that H. japonica increases its reproductive effort in the event that they die earlier due to Chytridiomycosis. This behavior would increase the chance of reproductive success by propagating their genes before they die.
Heterospecific amplexus
H. japonica have been observed with Pelophylax chosenicus in amplexus. Both species inhabit rice paddies and this shared habitat is a possible explanation for the observed interspecies copulation. Mating between different, but closely related species can sometimes result in hybridization. Further work is required to uncover the extent of heterospecific amplexus between H. japonica and Pelophylax chosenicus.
Predators
Predation by the American bullfrog
Lithobates catesbeianus, colloquially known as the American bullfrog, is an exotic predator of H. japonica. Predation by L. catesbeianus has been shown to significantly decrease the bone mineral density of H. japonica. Because bone mineral density can be used as a proxy for food intake, the conclusion that L. catesbeianus predation of H. japonica exerted a predation pressure that reduced food intake of H. japonica can be drawn. Predation by L. catesbeianus was not observed to induce any morphological changes in H. japonica.

Physiology
Predator defense by toxic peptide secretion
H. japonica have evolved against predation in arboreal environments by producing special Anntoxin-like neurotoxins from their skin. Anntoxin is a 60-residue toxic peptide that inhibits ion channels such as tetrodotoxin-sensitive voltage-gated sodium channels. While these peptides display analgesic properties after binding onto ion channels, they can harm and kill predators after frog skin consumption. Such a mechanism deters predators from further frog hunting.
Cold resistance
H. japonica demonstrates the remarkable ability to withstand extremely cold temperatures. H. japonica is able to survive temperatures as low as −35 °C. The majority of H. japonica individuals in a population from the Amur River were shown to withstand multiple rounds of exposure to −30 °C. These H. japonica were shown to survive at −30 °C for up to 120 days. Other frog species, at such temperatures, will accumulate ice, a phenomenon that proves lethal. This accumulation of ice was not observed in H. japonica.
During the exposure to cold, H. japonica seems to produce glycerol. This production of glycerol increases as temperature decreases. It is thought that this glycerol production plays a role in the cold-resistance of H. japonica. However, other frog species have similar glycerol production, but do not have cold resistance to the extent of H. japonica. Thus, the biochemical mechanism for the cold resistance of H. japonica is yet to be fully determined.
Bone mineral density
H. japonica have also been studied in order to determine the predictive ability of bone mineral density on the physiological well-being of frogs. Frogs with observed bone fractures on CT scan did not have significantly different bone mineral densities in comparison to healthy frogs. Thus, these frogs were unlikely to suffer from bone mineral diseases, and their fractures are more likely attributed to trauma-related injury.
Bone mineral density was strongly correlated to snout-vent length in H. japonica. Bone mineral density was not observed to be significantly different between males and females. This lack of difference can be attributed to the similar eating habits of both male and female H. japonica. H. japonica were observed to have fractures distributed similarly in both their forelimbs and hindlimbs.
Bone mineral density was able to effectively evaluate food status and physiological condition in H. japonica. This finding offers a mechanism for determination of food status for anuran populations.
Anuran vasotocin and mesotocin receptors
H. japonica has been used to determine the effects of anuran vasotocin (VT) and mesotocin (MT) receptors. VT, coupled to cyclic AMP, has antidiuretic effects in most amphibians. MT, which acts through the inositol/calcium signaling pathway induces diuretic effects in most amphibians. It was discovered that H. japonica contains both VT and MT receptors and that these receptors are differentially expressed in the body of the frog. VT receptors are localized to the pelvic patch of skin, whereas MT receptors are found in the fat body of the frog. Both MT and CT receptors are found in the brain, heart, kidney, and urinary bladder. This differential distribution of MT and VT receptors affects the cutaneous water absorption of H. japonica.
Conservation

The IUCN determined that the endangerment level of H. japonica is of "Least Concern". The population is listed as stable and non-fragmented. The IUCN lists some potential threats to H. japonica, which are primarily pollution and related to other environmental factors. Specifically, droughts that will occur at a higher frequency due to climate change will negatively affect the habitats of H. japonica as they rely on inland water to survive. In addition, increased agriculture and land for livestock may displace some H. japonica. H. japonica are reported to be able to survive in other habitats, such as rice paddies. Thus, the effects of this shift in potential habitat are unlikely to affect H. japonica due to the ability of H. japonica to survive in habitats ranging from urban to mountainous regions.
Additionally, H. Japonica tadpoles are susceptible to the ranavirus. Ranavirus transmits through animal-animal contact and has symptoms including abdominal edema, skin hemorrhaging, as well as damage to the liver, kidney, and spleen. Climate and habitat change have both contributed to increased virus transmission. Aside from tadpoles, ranavirus infects many amphibians, fish, and other cold-blood species.
Human application
H. japonica males will space their calls out such that males will avoid calling at the same time. This spacing out occurs in order to allow females to listen to each of the males' calls. In situations where multiple H. japonica males call at the same time, the female is unable to determine the location of each male calling. This makes mating difficult because the female has to be able to locate the male in order to mate. H. japonica males are able to desynchronize their calls with relatively little central organization or communication.
Humans have studied this ability of H. japonica males to behave in a coordinated manner despite no central organization or communication. Humans have used H. japonica observations in order to design wireless communication networks in order to improve efficiency in situations where no central communication hub is present. This area of science and development is termed "swarm intelligence" and further research is currently being conducted.
References
- Sergius Kuzmin; Irina Maslova; Masafumi Matsui; Fei Liang; Yoshio Kaneko (2017). "Dryophytes japonicus". IUCN Red List of Threatened Species. 2017: e.T55519A112714533. doi:10.2305/IUCN.UK.2017-1.RLTS.T55519A112714533.en.
- ^ Berman, D. I.; Meshcheryakova, E. N.; Bulakhova, N. A. (2016-11-01). "The Japanese tree frog (Hyla japonica), one of the most cold-resistant species of amphibians". Doklady Biological Sciences. 471 (1): 276–279. doi:10.1134/S0012496616060065. ISSN 1608-3105. PMID 28058600. S2CID 9770169.
- ^ IUCN (2004-04-30). "Dryophytes japonicus: Kuzmin, S., Maslova, I., Matsui, M., Liang, F. & Kaneko, Y.: The IUCN Red List of Threatened Species 2017: e.T55519A112714533". doi:10.2305/iucn.uk.2017-1.rlts.t55519a112714533.en.
{{cite journal}}: Cite journal requires|journal=(help) - Izumi-Kurotani, A.; Yamashita, M.; Kawasaki, Y.; Kurotani, T.; Mogami, Y.; Okuno, M.; Oketa, A.; Shiraishi, A.; Ueda, K.; Wassersug, R. J.; Naitoh, T. (1994-08-01). "Behavior of Japanese tree frogs under microgravity on MIR and in parabolic flight". Advances in Space Research. 14 (8): 419–422. Bibcode:1994AdSpR..14h.419I. doi:10.1016/0273-1177(94)90434-0. ISSN 0273-1177. PMID 11537951.
- ^ "AmphibiaWeb - Hyla japonica". amphibiaweb.org. Retrieved 2022-10-26.
- ^ ITIS (2022). "The Integrated Taxonomic Information System". doi:10.48580/dfq8-4ky.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Borzée, Amaёl; Park, Soyeon; Kim, Ahbin; Kim, Hyun-Tae; Jang, Yikweon (October 2013). "Morphometrics of two sympatric species of tree frogs in Korea: a morphological key for the critically endangered Hyla suweonensis in relation to H. japonica". Animal Cells and Systems. 17 (5): 348–356. doi:10.1080/19768354.2013.842931. ISSN 1976-8354. S2CID 83830853.
- ^ Kim, Eun-Bin; Kim, Eung-Sam; Sung, Ha-Cheol; Lee, Dong-Hyun; Kim, Geun-Joong; Nam, Dong-Ha (2021-06-01). "Comparison of the skeletal features of two sympatric tree frogs (Hylidae:Hyla)—Hyla japonica and Hyla suweonensis—using three-dimensional micro-computed tomography". Journal of Asia-Pacific Biodiversity. 14 (2): 147–153. doi:10.1016/j.japb.2021.03.002. ISSN 2287-884X. S2CID 233711067.
- ^ Hirai, Toshiaki; Matsui, Masafumi (1 September 2000). "Feeding Habits of the Japanese Tree Frog, Hyla japonica, in the Reproductive Season". Zoological Science. 17 (7): 977–982. doi:10.2108/zsj.17.977. hdl:2433/65049. ISSN 0289-0003. S2CID 86529597.
- ^ Kuramoto, Mitsuru (1980). "Mating Calls of Treefrogs (Genus Hyla) in the Far East, with Description of a New Species from Korea". Copeia. 1980 (1): 100–108. doi:10.2307/1444138. ISSN 0045-8511. JSTOR 1444138.
- ^ Maslova, Irina, et al. "Colour variants in the Japanese Treefrog (Dryophytes japonicus) from Russia and Korea." Herpetology Notes 11 (2018): 1007–1008.
- ^ Naito, Risa; Sakai, Masaru; Natuhara, Yosihiro; Morimoto, Yukihiro; Shibata, Shozo (1 May 2013). "Microhabitat use by Hyla japonica and Pelophylax porosa brevipoda at Levees in Rice Paddy Areas of Japan". Zoological Science. 30 (5): 386–391. doi:10.2108/zsj.30.386. ISSN 0289-0003. PMID 23646944. S2CID 6823482.
- ^ Izumi-Kurotani, A.; Yamashita, M.; Kawasaki, Y.; Kurotani, T.; Mogami, Y.; Okuno, M.; Oketa, A.; Shiraishi, A.; Ueda, K.; Wassersug, R. J.; Naitoh, T. (1994-08-01). "Behavior of Japanese tree frogs under microgravity on MIR and in parabolic flight". Advances in Space Research. 14 (8): 419–422. Bibcode:1994AdSpR..14h.419I. doi:10.1016/0273-1177(94)90434-0. ISSN 0273-1177. PMID 11537951.
- ^ 김준영 (2015). "Lekking behavior in the Japanese treefrog Hyla japonica". 이화여자대학교 대학원.
- ^ Borzée, Amaël; Purevdorj, Zoljargal; Kim, Ye Inn; Kong, Sungsik; Choe, Minjee; Yi, Yoonjung; Kim, Kyungmin; Kim, Ajoung; Jang, Yikweon (2019-11-25). "Breeding preferences in the treefrogs Dryophytes japonicus (Hylidae) in Mongolia". Journal of Natural History. 53 (43–44): 2685–2698. doi:10.1080/00222933.2019.1704458. ISSN 0022-2933. S2CID 213060965.
- ^ An, Deuknam; Waldman, Bruce (2016-03-31). "Enhanced call effort in Japanese tree frogs infected by amphibian chytrid fungus". Biology Letters. 12 (3): 20160018. doi:10.1098/rsbl.2016.0018. PMC 4843226. PMID 26932682.
- Bataille, Arnaud; Fong, Jonathan J.; Cha, Moonsuk; Wogan, Guinevere O. U.; Baek, Hae Jun; Lee, Hang; Min, Mi-Sook; Waldman, Bruce (17 Feb 2020). "Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians". Molecular Ecology. 22 (16): 4196–4209. doi:10.1111/mec.12385. PMID 23802586. S2CID 43246245.
- ^ Koo, Kyo Soung, et al. "First record of heterospecific amplexus behaviour between Pelophylax chosenicus (Okada, 1931) and Dryophytes japonicus (Günther, 1859) In Paju, Republic of Korea." Herpetology Notes 14 (2021): 1225–1226.
- ^ Park, Jun-Kyu; Do, Yuno (2020-07-15). "Evaluating the physical condition of Hyla japonica using radiographic techniques". Science of the Total Environment. 726: 138596. Bibcode:2020ScTEn.72638596P. doi:10.1016/j.scitotenv.2020.138596. ISSN 0048-9697. PMID 32305770. S2CID 216029647.
- ^ Chai, Longhui; Yin, Chuanlin; Kamau, Peter Muiruri; Luo, Lei; Yang, Shilong; Lu, Xiancui; Zheng, Dong; Wang, Yunfei (2021-09-01). "Toward an understanding of tree frog (Hyla japonica) for predator deterrence". Amino Acids. 53 (9): 1405–1413. doi:10.1007/s00726-021-03037-0. ISSN 1438-2199. PMID 34245370. S2CID 235786405.
- Wei, Lin; Dong, Li; Zhao, Tongyan; You, Dewen; Liu, Rui; Liu, Huan; Yang, Hailong; Lai, Ren (2011). "Analgesic and anti-inflammatory effects of the amphibian neurotoxin, anntoxin". Biochimie. 93 (6): 995–1000. doi:10.1016/j.biochi.2011.02.010. PMID 21376777.
- "Mesotocin - an overview | ScienceDirect Topics".
- ^ Kohno, Satomi; Kamishima, Yoshihisa; Iguchi, Taisen (2003-07-01). "Molecular cloning of an anuran V2 type [Arg8] vasotocin receptor and mesotocin receptor: functional characterization and tissue expression in the Japanese tree frog (Hyla japonica)". General and Comparative Endocrinology. 132 (3): 485–498. doi:10.1016/S0016-6480(03)00140-0. ISSN 0016-6480. PMID 12849972.
- ^ Park, Il Kook; Koo, Kyo-Soung; Moon, Kwang-Yeon; Lee, Jin-Gu; Park, Daesik. "PCR Detection of Ranavirus from Dead Kaloula borealis and Sick Hyla japonica Tadpoles in the Wild". Korean Journal of Herpetology: 10–14.
- Lesbarrères, D.; Balseiro, A.; Brunner, J.; Chinchar, V. G.; Duffus, A.; Kerby, J.; Miller, D. L.; Robert, J.; Schock, D. M.; Waltzek, T.; Gray, M. J. (2012-08-23). "Ranavirus: past, present and future". Biology Letters. 8 (4): 481–483. doi:10.1098/rsbl.2011.0951. PMC 3391431. PMID 22048891.
- ^ "Frog calls inspire a new algorithm for wireless networks". ScienceDaily. Retrieved 2022-10-26.
External links
 Data related to Hyla japonica at Wikispecies
Data related to Hyla japonica at Wikispecies Media related to Hyla japonica at Wikimedia Commons
Media related to Hyla japonica at Wikimedia Commons
| Taxon identifiers | |
|---|---|
| Hyla japonica | |