Medical condition
| Rabies | |
|---|---|
 | |
| A man with rabies, 1958 | |
| Specialty | Infectious disease |
| Symptoms | Fever, extreme aversion to water, confusion, excessive salivary secretion, hallucinations, disrupted sleep, paralysis, coma, hyperactivity, headache, nausea, vomiting, anxiety |
| Causes | Rabies virus, Australian bat lyssavirus |
| Prevention | Rabies vaccine, animal control, rabies immunoglobulin |
| Treatment | Supportive care |
| Medication | Incurable |
| Prognosis | ~100% fatal after onset of symptoms |
| Deaths | 59,000 per year worldwide |
Rabies is a viral disease that causes encephalitis in humans and other mammals. It was historically referred to as hydrophobia ("fear of water") because its victims would panic when offered liquids to drink. Early symptoms can include fever and abnormal sensations at the site of exposure. These symptoms are followed by one or more of the following symptoms: nausea, vomiting, violent movements, uncontrolled excitement, fear of water, an inability to move parts of the body, confusion, and loss of consciousness. Once symptoms appear, the result is virtually always death. The time period between contracting the disease and the start of symptoms is usually one to three months but can vary from less than one week to more than one year. The time depends on the distance the virus must travel along peripheral nerves to reach the central nervous system.
Rabies is caused by lyssaviruses, including the rabies virus and Australian bat lyssavirus. It is spread when an infected animal bites or scratches a human or other animals. Saliva from an infected animal can also transmit rabies if the saliva comes into contact with the eyes, mouth, or nose. Globally, dogs are the most common animal involved. In countries where dogs commonly have the disease, more than 99% of rabies cases in humans are the direct result of dog bites. In the Americas, bat bites are the most common source of rabies infections in humans, and less than 5% of cases are from dogs. Rodents are very rarely infected with rabies. The disease can be diagnosed only after the start of symptoms.
Animal control and vaccination programs have decreased the risk of rabies from dogs in a number of regions of the world. Immunizing people before they are exposed is recommended for those at high risk, including those who work with bats or who spend prolonged periods in areas of the world where rabies is common. In people who have been exposed to rabies, the rabies vaccine and sometimes rabies immunoglobulin are effective in preventing the disease if the person receives the treatment before the start of rabies symptoms. Washing bites and scratches for 15 minutes with soap and water, povidone-iodine, or detergent may reduce the number of viral particles and may be somewhat effective at preventing transmission. As of 2016, only fourteen people were documented to have survived a rabies infection after showing symptoms. However, research conducted in 2010 among a population of people in Peru with a self-reported history of one or more bites from vampire bats (commonly infected with rabies), found that out of 73 individuals reporting previous bat bites, seven people had rabies virus-neutralizing antibodies (rVNA). Since only one member of this group reported prior vaccination for rabies, the findings of the research suggest previously undocumented cases of infection and viral replication followed by an abortive infection. This could indicate that people may have an exposure to the virus without treatment and develop natural antibodies as a result.
Rabies causes about 59,000 deaths worldwide per year, about 40% of which are in children under the age of 15. More than 95% of human deaths from rabies occur in Africa and Asia. Rabies is present in more than 150 countries and on all continents but Antarctica. More than 3 billion people live in regions of the world where rabies occurs. A number of countries, including Australia and Japan, as well as much of Western Europe, do not have rabies among dogs. Many Pacific islands do not have rabies at all. It is classified as a neglected tropical disease.
The global cost of rabies is estimated to be around US$8.6 billion per year including lost lives and livelihoods, medical care and associated costs, as well as uncalculated psychological trauma.
Etymology
The name rabies is derived from the Latin rabies, meaning "madness". The Greeks derived the word lyssa, from lud or "violent"; this root is used in the genus name of the rabies virus, Lyssavirus.
Signs and symptoms

The period between infection and the first symptoms (incubation period) is typically one to three months in humans. This period may be as short as four days or longer than six years, depending on the location and severity of the wound and the amount of virus introduced. Initial symptoms of rabies are often nonspecific, such as fever and headache. As rabies progresses and causes inflammation of the brain and meninges, symptoms can include slight or partial paralysis, anxiety, insomnia, confusion, agitation, abnormal behavior, paranoia, terror, and hallucinations. The person may also have fear of water.
The symptoms eventually progress to delirium and coma. Death usually occurs two to ten days after first symptoms. Survival is almost unknown once symptoms have presented, even with intensive care.
Rabies has also occasionally been referred to as hydrophobia ("fear of water") throughout its history. It refers to a set of symptoms in the later stages of an infection in which the person has difficulty swallowing, shows panic when presented with liquids to drink, and cannot quench their thirst. Saliva production is greatly increased, and attempts to drink, or even the intention or suggestion of drinking, may cause excruciatingly painful spasms of the muscles in the throat and larynx. Since the infected individual cannot swallow saliva and water, the virus has a much higher chance of being transmitted, because it multiplies and accumulates in the salivary glands and is transmitted through biting.
Hydrophobia is commonly associated with furious rabies, which affects 80% of rabies-infected people. This form of rabies causes irrational aggression in the host, which aids in the spreading of the virus through animal bites; a "foaming at the mouth" effect, caused by the accumulation of saliva, is also commonly associated with rabies in the public perception and in popular culture. The remaining 20% may experience a paralytic form of rabies that is marked by muscle weakness, loss of sensation, and paralysis; this form of rabies does not usually cause fear of water.
Cause
-
 Rendering of the rabies virus
Rendering of the rabies virus
-
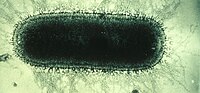 An electron microscope image of the rabies virus
An electron microscope image of the rabies virus
-
TEM micrograph with numerous rabies virions (small, dark grey, rodlike particles) and Negri bodies (the larger pathognomonic cellular inclusions of rabies infection)
Rabies is caused by a number of lyssaviruses including the rabies virus and Australian bat lyssavirus. Duvenhage lyssavirus may cause a rabies-like infection.
The rabies virus is the type species of the Lyssavirus genus, in the family Rhabdoviridae, order Mononegavirales. Lyssavirions have helical symmetry, with a length of about 180 nm and a cross-section of about 75 nm. These virions are enveloped and have a single-stranded RNA genome with negative sense. The genetic information is packed as a ribonucleoprotein complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome of the virus encodes five genes whose order is highly conserved: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and the viral RNA polymerase (L).
To enter cells, trimeric spikes on the exterior of the membrane of the virus interact with a specific cell receptor, the most likely one being the acetylcholine receptor. The cellular membrane pinches in a procession known as pinocytosis and allows entry of the virus into the cell by way of an endosome. The virus then uses the acidic environment, which is necessary, of that endosome and binds to its membrane simultaneously, releasing its five proteins and single-strand RNA into the cytoplasm.
Once within a muscle or nerve cell, the virus undergoes replication. The L protein then transcribes five mRNA strands and a positive strand of RNA all from the original negative strand RNA using free nucleotides in the cytoplasm. These five mRNA strands are then translated into their corresponding proteins (P, L, N, G and M proteins) at free ribosomes in the cytoplasm. Some proteins require post-translational modifications. For example, the G protein travels through the rough endoplasmic reticulum, where it undergoes further folding, and is then transported to the Golgi apparatus, where a sugar group is added to it (glycosylation).
When there are enough viral proteins, the viral polymerase will begin to synthesize new negative strands of RNA from the template of the positive-strand RNA. These negative strands will then form complexes with the N, P, L and M proteins and then travel to the inner membrane of the cell, where a G protein has embedded itself in the membrane. The G protein then coils around the N-P-L-M complex of proteins taking some of the host cell membrane with it, which will form the new outer envelope of the virus particle. The virus then buds from the cell.
From the point of entry, the virus is neurotropic, traveling along the neural pathways into the central nervous system. The virus usually first infects muscle cells close to the site of infection, where they are able to replicate without being 'noticed' by the host's immune system. Once enough virus has been replicated, they begin to bind to acetylcholine receptors at the neuromuscular junction. The virus then travels through the nerve cell axon via retrograde transport, as its P protein interacts with dynein, a protein present in the cytoplasm of nerve cells. Once the virus reaches the cell body it travels rapidly to the central nervous system (CNS), replicating in motor neurons and eventually reaching the brain. After the brain is infected, the virus travels centrifugally to the peripheral and autonomic nervous systems, eventually migrating to the salivary glands, where it is ready to be transmitted to the next host.
Transmission
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. Birds were first artificially infected with rabies in 1884; however, infected birds are largely, if not wholly, asymptomatic, and recover. Other bird species have been known to develop rabies antibodies, a sign of infection, after feeding on rabies-infected mammals.
The virus has also adapted to grow in cells of cold-blooded vertebrates. Most animals can be infected by the virus and can transmit the disease to humans. Worldwide, about 99% of human rabies cases come from domestic dogs. Other sources of rabies in humans include bats, monkeys, raccoons, foxes, skunks, cattle, wolves, coyotes, cats, and mongooses (normally either the small Asian mongoose or the yellow mongoose).
Rabies may also spread through exposure to infected bears, domestic farm animals, groundhogs, weasels, and other wild carnivorans. However, lagomorphs, such as hares and rabbits, and small rodents, such as chipmunks, gerbils, guinea pigs, hamsters, mice, rats, and squirrels, are almost never found to be infected with rabies and are not known to transmit rabies to humans. Bites from mice, rats, or squirrels rarely require rabies prevention because these rodents are typically killed by any encounter with a larger, rabid animal, and would, therefore, not be carriers. The Virginia opossum (a marsupial, unlike the other mammals named in this paragraph, which are all eutherians/placental), has a lower internal body temperature than the rabies virus prefers and therefore is resistant but not immune to rabies. Marsupials, along with monotremes (platypuses and echidnas), typically have lower body temperatures than similarly sized eutherians. In 2024, reports emerged that rabies is spreading in South African seals, possibly making it the first outbreak documented in marine mammals.
The virus is usually present in the nerves and saliva of a symptomatic rabid animal. The route of infection is usually, but not always, by a bite. In many cases, the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behavior. This is an example of a viral pathogen modifying the behavior of its host to facilitate its transmission to other hosts. After a typical human infection by bite, the virus enters the peripheral nervous system. It then travels retrograde along the efferent nerves toward the central nervous system. During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the brain, it rapidly causes encephalitis, the prodromal phase, which is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the spinal cord, producing transverse myelitis.
Although it is theoretically possible for rabies-infected humans to transmit it to others by biting or otherwise, no such cases have ever been documented, because infected humans are usually hospitalized and necessary precautions taken. Casual contact, such as touching a person with rabies or contact with non-infectious fluid or tissue (urine, blood, feces), does not constitute an exposure and does not require post-exposure prophylaxis. But as the virus is present in sperm and vaginal secretions, it might be possible for rabies to spread through sex. There are only a small number of recorded cases of human-to-human transmission of rabies, and all occurred through organ transplants, most frequently with corneal transplantation, from infected donors.
Diagnosis
Rabies can be difficult to diagnose because, in the early stages, it is easily confused with other diseases or even with a simple aggressive temperament. The reference method for diagnosing rabies is the fluorescent antibody test (FAT), an immunohistochemistry procedure, which is recommended by the World Health Organization (WHO). The FAT relies on the ability of a detector molecule (usually fluorescein isothiocyanate) coupled with a rabies-specific antibody, forming a conjugate, to bind to and allow the visualisation of rabies antigen using fluorescent microscopy techniques. Microscopic analysis of samples is the only direct method that allows for the identification of rabies virus-specific antigen in a short time and at a reduced cost, irrespective of geographical origin and status of the host. It has to be regarded as the first step in diagnostic procedures for all laboratories. Autolysed samples can, however, reduce the sensitivity and specificity of the FAT. The RT PCR assays proved to be a sensitive and specific tool for routine diagnostic purposes, particularly in decomposed samples or archival specimens. The diagnosis can be reliably made from brain samples taken after death. The diagnosis can also be made from saliva, urine, and cerebrospinal fluid samples, but this is not as sensitive or reliable as brain samples. Cerebral inclusion bodies called Negri bodies are 100% diagnostic for rabies infection but are found in only about 80% of cases. If possible, the animal from which the bite was received should also be examined for rabies.
Some light microscopy techniques may also be used to diagnose rabies at a tenth of the cost of traditional fluorescence microscopy techniques, allowing identification of the disease in less-developed countries. A test for rabies, known as LN34, is easier to run on a dead animal's brain and might help determine who does and does not need post-exposure prevention. The test was developed by the Centers for Disease Control and Prevention (CDC) in 2018.
The differential diagnosis in a case of suspected human rabies may initially include any cause of encephalitis, in particular infection with viruses such as herpesviruses, enteroviruses, and arboviruses such as West Nile virus. The most important viruses to rule out are herpes simplex virus type one, varicella zoster virus, and (less commonly) enteroviruses, including coxsackieviruses, echoviruses, polioviruses, and human enteroviruses 68 to 71.
New causes of viral encephalitis are also possible, as was evidenced by the 1999 outbreak in Malaysia of 300 cases of encephalitis with a mortality rate of 40% caused by Nipah virus, a newly recognized paramyxovirus. Likewise, well-known viruses may be introduced into new locales, as is illustrated by the outbreak of encephalitis due to West Nile virus in the eastern United States.
Prevention
Further information: Dog bite preventionAlmost all human exposure to rabies was fatal until a vaccine was developed in 1885 by Louis Pasteur and Émile Roux. Their original vaccine was harvested from infected rabbits, from which the virus in the nerve tissue was weakened by allowing it to dry for five to ten days. Similar nerve tissue-derived vaccines are still used in some countries, as they are much cheaper than modern cell culture vaccines.
The human diploid cell rabies vaccine was started in 1967. Less expensive purified chicken embryo cell vaccine and purified vero cell rabies vaccine are now available. A recombinant vaccine called V-RG has been used in Belgium, France, Germany, and the United States to prevent outbreaks of rabies in undomesticated animals. Immunization before exposure has been used in both human and nonhuman populations, where, as in many jurisdictions, domesticated animals are required to be vaccinated.

The Missouri Department of Health and Senior Services Communicable Disease Surveillance 2007 Annual Report states the following can help reduce the risk of contracting rabies:
- Vaccinating dogs, cats, and ferrets against rabies
- Keeping pets under supervision
- Not handling wild animals or strays
- Contacting an animal control officer upon observing a wild animal or a stray, especially if the animal is acting strangely
- If bitten by an animal, washing the wound with soap and water for 10 to 15 minutes and contacting a healthcare provider to determine if post-exposure prophylaxis is required
28 September is World Rabies Day, which promotes the information, prevention, and elimination of the disease.
In Asia and in parts of the Americas and Africa, dogs remain the principal host. Mandatory vaccination of animals is less effective in rural areas. Especially in developing countries, pets may not be privately kept and their destruction may be unacceptable. Oral vaccines can be safely distributed in baits, a practice that has successfully reduced rabies in rural areas of Canada, France, and the United States. In Montreal, Quebec, Canada, baits are successfully used on raccoons in the Mount-Royal Park area. Vaccination campaigns may be expensive, but cost-benefit analysis suggests baits may be a cost-effective method of control. In Ontario, a dramatic drop in rabies was recorded when an aerial bait-vaccination campaign was launched.
The number of recorded human deaths from rabies in the United States has dropped from 100 or more annually in the early 20th century to one or two per year because of widespread vaccination of domestic dogs and cats and the development of human vaccines and immunoglobulin treatments. Most deaths now result from bat bites, which may go unnoticed by the victim and hence untreated.
Treatment
After exposure
Treatment after exposure can prevent the disease if given within 10 days. The rabies vaccine is 100% effective if given before symptoms of rabies appear. Every year, more than 15 million people get vaccinated after potential exposure. While this works well, the cost is significant. In the US it is recommended people receive one dose of human rabies immunoglobulin (HRIG) and four doses of rabies vaccine over a 14-day period. HRIG is expensive and makes up most of the cost of post-exposure treatment, ranging as high as several thousand dollars. In the UK, one dose of HRIG costs the National Health Service £1,000, although this is not flagged as a "high-cost medication". A full course of vaccine costs £120–180. As much as possible of HRIG should be injected around the bites, with the remainder being given by deep intramuscular injection at a site distant from the vaccination site.
People who have previously been vaccinated against rabies do not need to receive the immunoglobulin—only the postexposure vaccinations on days 0 and 3. The side effects of modern cell-based vaccines are similar to the side effects of flu shots. The old nerve-tissue-based vaccination required multiple injections into the abdomen with a large needle but is inexpensive. It is being phased out and replaced by affordable World Health Organization intradermal-vaccination regimens. In children less than a year old, the lateral thigh is recommended.
Thoroughly washing the wound as soon as possible with soap and water for approximately five minutes is effective in reducing the number of viral particles. Povidone-iodine or alcohol is then recommended to reduce the virus further.
Awakening to find a bat in the room, or finding a bat in the room of a previously unattended child or mentally disabled or intoxicated person, is an indication for post-exposure prophylaxis (PEP). The recommendation for the precautionary use of PEP in bat encounters where no contact is recognized has been questioned in the medical literature, based on a cost–benefit analysis. However, a 2002 study has supported the protocol of precautionary administration of PEP where a child or mentally compromised individual has been alone with a bat, especially in sleep areas, where a bite or exposure may occur with the victim being unaware.
After onset
Once rabies develops, death almost certainly follows. Palliative care in a hospital setting is recommended with administration of large doses of pain medication, and sedatives in preference to physical restraint. Ice fragments can be given by mouth for thirst, but there is no good evidence intravenous hydration is of benefit.
A treatment known as the Milwaukee protocol, which involves putting people with rabies symptoms into a chemically induced coma and using antiviral medications in an attempt to protect their brain until their body has had time to produce rabies antibodies, has been occasionally used. It was initially attempted in 2004 on Jeanna Giese, a teenage girl from Wisconsin, who subsequently became the first human known to have survived rabies without receiving post-exposure prophylaxis before symptom onset. Giese did require extensive rehabilitation afterward, and her balance and neural function remained impaired. The protocol has been enacted on many rabies victims since, but has been adjudged a failure; some survivors of the acute initial phase later died of rabies. Concerns have also been raised about its monetary costs and its ethics.
Antiviral therapy to combat the effects of rabies has also been researched; favipiravir, for example, has shown potential at inhibiting the development of encephalitis. It has been suggested that such therapy in combination with immunotherapy and neuroprotective measures could be beneficial.
Prognosis
Vaccination after exposure, PEP, is highly successful in preventing rabies. In unvaccinated humans, rabies is almost certainly fatal after neurological symptoms have developed.
Epidemiology
Main article: Prevalence of rabies
In 2010, an estimated 26,000 people died from rabies, down from 54,000 in 1990. The majority of the deaths occurred in Asia and Africa. As of 2015, India (approximately 20,847), followed by China (approximately 6,000) and the Democratic Republic of the Congo (5,600), had the most cases. A 2015 collaboration between the World Health Organization, World Organization of Animal Health (OIE), Food and Agriculture Organization of the United Nation (FAO), and Global Alliance for Rabies Control has a goal of eliminating deaths from rabies by 2030.
India
India has the highest rate of human rabies in the world, primarily because of stray dogs, whose number has greatly increased since a 2001 law forbade the killing of dogs. Effective control and treatment of rabies in India is hindered by a form of mass hysteria known as puppy pregnancy syndrome (PPS). Dog bite victims with PPS, male as well as female, become convinced that puppies are growing inside them, and often seek help from faith healers rather than medical services. An estimated 20,000 people die every year from rabies in India, more than a third of the global total.
Australia
Australia has an official rabies-free status, although Australian bat lyssavirus (ABLV), discovered in 1996, is a rabies-causing virus related to the rabies virus prevalent in Australian native bat populations.
United States

Canine-specific rabies has been eradicated in the United States, but rabies is common among wild animals, and an average of 100 dogs become infected from other wildlife each year.
High public awareness of the virus, efforts at vaccination of domestic animals and curtailment of feral populations, and availability of postexposure prophylaxis have made rabies very rare in humans in the United States. From 1960 to 2018, a total of 125 such cases were reported in the United States; of them, 36 (28%) were attributed to dog bites suffered during international travel. Among the 89 infections acquired in the United States, 62 (70%) were attributed to bats. The most recent rabies death in the United States was in November 2021, where a Texas child was bitten by a bat in late August 2021 but his parents failed to get him treatment. He died less than three months later.
Europe
Either no or very few cases of rabies are reported each year in Europe; cases are contracted both during travel and in Europe.
In Switzerland the disease was virtually eliminated after scientists placed chicken heads laced with live attenuated vaccine in the Swiss Alps. Foxes, proven to be the main source of rabies in the country, ate the chicken heads and became immunized.
Italy, after being declared rabies-free from 1997 to 2008, has witnessed a reemergence of the disease in wild animals in the Triveneto regions (Trentino-Alto Adige/Südtirol, Veneto and Friuli-Venezia Giulia) due to the spreading of an epidemic in the Balkans that also affected Austria. An extensive wild animal vaccination campaign eliminated the virus from Italy again, and it regained the rabies-free country status in 2013, the last reported case of rabies being reported in a red fox in early 2011.
The United Kingdom has been free of rabies since the early 20th century except for a rabies-like virus (EBLV-2) in a few Daubenton's bats. There has been one fatal case of EBLV-2 transmission to a human. There have been four deaths from rabies, transmitted abroad by dog bites, since 2000. The last infection in the UK occurred in 1922, and the last death from indigenous rabies was in 1902.
Sweden and mainland Norway have been free of rabies since 1886. Bat rabies antibodies (but not the virus) have been found in bats. On Svalbard, animals can cross the arctic ice from Greenland or Russia.
Mexico
Mexico was certified by the World Health Organization as being free of dog-transmitted rabies in 2019 because no case of dog-human transmission had been recorded in two years.
Asian countries
Despite rabies being preventable and the many successes of the years from countries such as North America, South Korea and Western Europe, Rabies remains endemic in many Southeast Asian countries including Cambodia, Bangladesh, Bhutan, North Korea, India, Indonesia, Myanmar Nepal, Sri Lanka, and Thailand. Half the global rabies deaths occur in southeast Asia - approx. 26,000 per year.
Much of what prevents Asia from implementing the same measures as other countries is cost. Treating wild canines is the primary means of preventing rabies, however it costs 10 times more than treating individuals as they come with bites, and research also increases cost. As a result, India and other surrounding countries are unable to apply many preventative measures because of financial restrictions.
Thailand
In 2013 human rabies was nearly eradicated in the state of Thailand after new measures were put into place requiring the vaccination of all domestic dogs as well as programs seeking to vaccinate wild dogs and large animals. However, neighboring countries unable to afford rabies control measures – Cambodia, Laos, and Myanmar – allowed infected animals to continue to pass the border and infect the Thai population, leading to ~100 cases a year. These areas around the border are called Rabies Red areas and are where Thailand continuously struggles with eradication and will do so until the surrounding countries eliminate the virus.
Thailand has the resources and medicine necessary to tackle rabies such as implementing regulations that require all children to receive a rabies vaccination before attending schools and having clinics available for those bit or scratched by a possible rabid animal. However, individual choice is still a factor, and 10 people per year die out of refusal to seek treatment.
Cambodia
Cambodia has approximately 800 cases of human rabies per year, making it one of the top countries in human rabies incidences. Much of this falls on their lack of animal care; Cambodia has hundreds of thousands of animals infected with rabies, another global high, yet little surveillance of said animals and few laws requiring pets and other household animals to be vaccinated. In recent years Cambodia has improved significantly in their human rabies medical practices, with clinics all over the countries being made with treatments and vaccination on hand as well as rabies-related education in school classes. However, it is still lacking in terms of animal surveillance and treatment which leads to bleeding into surrounding countries.
History
Rabies has been known since around 2000 BC. The first written record of rabies is in the Mesopotamian Codex of Eshnunna (c. 1930 BC), which dictates that the owner of a dog showing symptoms of rabies should take preventive measures against bites. If another person were bitten by a rabid dog and later died, the owner was heavily fined.
In ancient Greece, rabies was supposed to be caused by Lyssa, the spirit of mad rage.
Ineffective folk remedies abounded in the medical literature of the ancient world. The physician Scribonius Largus prescribed a poultice of cloth and hyena skin; Antaeus recommended a preparation made from the skull of a hanged man.
Rabies appears to have originated in the Old World, the first epizootic in the New World occurring in Boston in 1768.
Rabies was considered a scourge for its prevalence in the 19th century. In France and Belgium, where Saint Hubert was venerated, the "St Hubert's Key" was heated and applied to cauterize the wound. By an application of magical thinking, dogs were branded with the key in hopes of protecting them from rabies.
It was not uncommon for a person bitten by a dog merely suspected of being rabid to commit suicide or to be killed by others.
In ancient times the attachment of the tongue (the lingual frenulum, a mucous membrane) was cut and removed, as this was where rabies was thought to originate. This practice ceased with the discovery of the actual cause of rabies. Louis Pasteur's 1885 nerve tissue vaccine was successful, and was progressively improved to reduce often severe side-effects.
In modern times, the fear of rabies has not diminished, and the disease and its symptoms, particularly agitation, have served as an inspiration for several works of zombie or similarly themed fiction, often portraying rabies as having mutated into a stronger virus which fills humans with murderous rage or incurable illness, bringing about a devastating, widespread pandemic.
-
 Miniature of the Cantiga #275 depicting two monks hospitaller with rabies being carried before St. Mary of Terena
Miniature of the Cantiga #275 depicting two monks hospitaller with rabies being carried before St. Mary of Terena
-
 A woodcut from the Middle Ages showing a rabid dog
A woodcut from the Middle Ages showing a rabid dog
-
 François Boissier de Sauvages de Lacroix, Della natura e causa della rabbia (Dissertation sur la nature et la cause de la Rage), 1777
François Boissier de Sauvages de Lacroix, Della natura e causa della rabbia (Dissertation sur la nature et la cause de la Rage), 1777
-
 An Arabic folio from the medieval times depicting a rabid dog biting a man, c. 1224
An Arabic folio from the medieval times depicting a rabid dog biting a man, c. 1224
Other animals
Main article: Rabies in animals
Rabies is infectious to mammals; three stages of central nervous system infection are recognized. The clinical course is often shorter in animals than in humans, but result in similar symptoms and almost always death. The first stage is a one- to three-day period characterized by behavioral changes and is known as the prodromal stage. The second is the excitative stage, which lasts three to four days. This stage is often known as "furious rabies" for the tendency of the affected animal to be hyper-reactive to external stimuli and bite or attack anything near. In some cases, animals skip the excitative stage and develop paralysis, as in the third phase; the paralytic phase. This stage develops by damage to motor neurons. Incoordination is seen, owing to rear limb paralysis, and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by respiratory arrest.
See also
References
- ^ "Rabies Fact Sheet N°99". World Health Organization. July 2013. Archived from the original on 1 April 2014. Retrieved 28 February 2014.
- "Rabies – Symptoms and causes". Mayo Clinic. Retrieved 9 April 2018.
- "Rabies". Mayo Clinic. Retrieved 5 October 2023.
- ^ "Rabies, Australian bat lyssavirus and other lyssaviruses". The Department of Health. December 2013. Archived from the original on 4 March 2014. Retrieved 1 March 2014.
- "Rabies diagnosis and treatment". Mayo clinic. 27 June 2023.
- ^ "Rabies". CDC. 29 July 2020. Retrieved 31 January 2021.
- "Rabies, Symptoms, Causes, Treatment". Medical News Today. 15 November 2017.
- "Rabies, Symptoms and causes". Mayo Clinic.
- "Animal bites and rabies". Johns Hopkins Medicine. 27 February 2020.
- ^ Cotran RS, Kumar V, Fausto N (2005). Robbins and Cotran Pathologic Basis of Disease (7th ed.). Elsevier/Saunders. p. 1375. ISBN 978-0-7216-0187-8.
- ^ Tintinalli JE (2010). Emergency Medicine: A Comprehensive Study Guide (Emergency Medicine (Tintinalli)). McGraw-Hill. pp. Chapter 152. ISBN 978-0-07-148480-0.
- Wunner WH (2010). Rabies: Scientific Basis of the Disease and Its Management. Academic Press. p. 556. ISBN 978-0-08-055009-1.
- Manoj S, Mukherjee A, Johri S, Kumar KV (2016). "Recovery from rabies, a universally fatal disease". Military Medical Research. 3 (1): 21. doi:10.1186/s40779-016-0089-y. ISSN 2054-9369. PMC 4947331. PMID 27429788.
- Weyer J, Msimang-Dermaux V, Paweska JT, Blumberg LH, Le Roux K, Govender P, et al. (9 June 2016). "A case of human survival of rabies, South Africa". Southern African Journal of Infectious Diseases. 31 (2): 66–68. doi:10.1080/23120053.2016.1128151. hdl:2263/59121. ISSN 2312-0053.
- Gilbert AT, Petersen BW, Recuenco S, Niezgoda M, Gómez J, Laguna-Torres VA, Rupprecht C (August 2012). "Evidence of rabies virus exposure among humans in the Peruvian Amazon". The American Journal of Tropical Medicine and Hygiene. 87 (2): 206–215. doi:10.4269/ajtmh.2012.11-0689. PMC 3414554. PMID 22855749.
- "Rabies: The Facts" (PDF). World Health Organization. Archived (PDF) from the original on 24 February 2017. Retrieved 24 February 2017.
- WHO Expert Consultation on Rabies : second report (PDF) (2nd ed.). Geneva: WHO. 2013. p. 3. ISBN 978-92-4-120982-3. Archived (PDF) from the original on 20 October 2014.
- ^ "Rabies-Free Countries and Political Units". CDC. Archived from the original on 5 March 2014. Retrieved 8 May 2019.
- "Neglected Tropical Diseases". cdc.gov. 6 June 2011. Archived from the original on 4 December 2014. Retrieved 28 November 2014.
- "Rabies". www.who.int. Retrieved 13 August 2024.
- Simpson DP (1979). Cassell's Latin Dictionary (5th ed.). London: Cassell. p. 883. ISBN 978-0-304-52257-6.
- ^ Rotivel Y (1995). Rupprecht CE, Smith JS, Fekadu M, Childs JE (eds.). "Introduction: The ascension of wildlife rabies: a cause for public health concern or intervention?". Emerging Infectious Diseases. 1 (4): 107–114. doi:10.3201/eid0104.950401. PMC 2626887. PMID 8903179. Archived from the original on 26 April 2009.
- ^ Giesen A, Gniel D, Malerczyk C (March 2015). "30 Years of rabies vaccination with Rabipur: a summary of clinical data and global experience". Expert Review of Vaccines (Review). 14 (3): 351–367. doi:10.1586/14760584.2015.1011134. PMID 25683583.
- Rupprecht CE, Willoughby R, Slate D (December 2006). "Current and future trends in the prevention, treatment and control of rabies". Expert Review of Anti-Infective Therapy. 4 (6): 1021–38. doi:10.1586/14787210.4.6.1021. PMID 17181418. S2CID 36979186.
- Cliff AD, Haggett P, Smallman-Raynor M (2004). World atlas of epidemic diseases. London: Arnold. p. 51. ISBN 978-0-340-76171-7.
- "Rabies". AnimalsWeCare.com. Archived from the original on 3 September 2014.
- "How does rabies cause aggression?". medicalnewstoday.com. Medical News Today. 14 October 2017. Retrieved 19 January 2023.
- Tian Z, Chen Y, Yan W (December 2019). "Clinical features of rabies patients with abnormal sexual behaviors as the presenting manifestations: a case report and literature review". BMC Infectious Diseases. 19 (1): 679. doi:10.1186/s12879-019-4252-4. ISSN 1471-2334. PMC 6670183. PMID 31370800.
- Wilson PJ, Rohde RE, Oertli EH, Willoughby Jr RE (2019). Rabies: Clinical Considerations and Exposure Evaluations. Elsevier. p. 28. ISBN 978-0-323-63979-8. Retrieved 10 May 2023.
- CDC. "How Do You Know if an Animal Has Rabies?". CDC Rabies and Kids. Centers for Disease Control and Prevention. Retrieved 10 May 2023.
- "Rabies (for Parents)". KidsHealth.org. Nemours KidsHealth. Retrieved 10 May 2023.
- "Symptoms of rabies". NHS.uk. 12 June 2012. Archived from the original on 14 September 2014. Retrieved 3 September 2014.
- van Thiel PP, de Bie RM, Eftimov F, Tepaske R, Zaaijer HL, van Doornum GJ, et al. (July 2009). "Fatal human rabies due to Duvenhage virus from a bat in Kenya: failure of treatment with coma-induction, ketamine, and antiviral drugs". PLOS Neglected Tropical Diseases. 3 (7): e428. doi:10.1371/journal.pntd.0000428. PMC 2710506. PMID 19636367.
- ^ Drew WL (2004). "Chapter 41: Rabies". In Ryan KJ, Ray CG (eds.). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 597–600. ISBN 978-0-8385-8529-0.
- Finke S, Conzelmann KK (August 2005). "Replication strategies of rabies virus". Virus Research. 111 (2): 120–131. doi:10.1016/j.virusres.2005.04.004. PMID 15885837.
- ^ "Rabies Post-Exposure Prophylaxis". Centers for Disease Control and Prevention (CDC). 23 December 2009. Archived from the original on 1 February 2010. Retrieved 30 January 2010.
- Gluska S, Zahavi EE, Chein M, Gradus T, Bauer A, Finke S, Perlson E (August 2014). "Rabies Virus Hijacks and accelerates the p75NTR retrograde axonal transport machinery". PLOS Pathogens. 10 (8): e1004348. doi:10.1371/journal.ppat.1004348. PMC 4148448. PMID 25165859.
- ^ Baer G (1991). The Natural History of Rabies. CRC Press. ISBN 978-0-8493-6760-1. Retrieved 31 October 2011.
- Shannon LM, Poulton JL, Emmons RW, Woodie JD, Fowler ME (April 1988). "Serological survey for rabies antibodies in raptors from California". Journal of Wildlife Diseases. 24 (2): 264–67. doi:10.7589/0090-3558-24.2.264. PMID 3286906.
- Gough PM, Jorgenson RD (July 1976). "Rabies antibodies in sera of wild birds". Journal of Wildlife Diseases. 12 (3): 392–5. doi:10.7589/0090-3558-12.3.392. PMID 16498885.
- Jorgenson RD, Gough PM, Graham DL (July 1976). "Experimental rabies in a great horned owl". Journal of Wildlife Diseases. 12 (3): 444–47. doi:10.7589/0090-3558-12.3.444. PMID 16498892. S2CID 11374356.
- Wong D. "Rabies". Wong's Virology. Archived from the original on 3 December 2008. Retrieved 19 March 2009.
- Campbell JB, Charlton K (1988). Developments in Veterinary Virology: Rabies. Springer. p. 48. ISBN 978-0-89838-390-4.
- "Rabies". World Health Organization. 21 April 2020. Retrieved 3 May 2021.
- Pawan JL (1959). "The transmission of paralytic rabies in Trinidad by the vampire bat (Desmodus rotundus murinus) Wagner". Caribbean Medical Journal. 21: 110–136. PMID 13858519.
- Pawan JL (1959). "Rabies in the vampire bat of Trinidad, with special reference to the clinical course and the latency of infection". Caribbean Medical Journal. 21: 137–156. PMID 14431118.
- Taylor PJ (December 1993). "A systematic and population genetic approach to the rabies problem in the yellow mongoose (Cynictis penicillata)". The Onderstepoort Journal of Veterinary Research. 60 (4): 379–387. PMID 7777324.
- "Rabies. Other Wild Animals: Terrestrial carnivores: raccoons, skunks and foxes". Centers for Disease Control and Prevention. Archived from the original on 20 December 2010. Retrieved 23 December 2010.
- Anderson J, Frey R (2006). "Rabies". In Fundukian LJ (ed.). Gale Encyclopedia of Medicine (3rd ed.).
- McRuer DL, Jones KD (May 2009). "Behavioral and nutritional aspects of the Virginian opossum (Didelphis virginiana)". The Veterinary Clinics of North America. Exotic Animal Practice. 12 (2): 217–236, viii. doi:10.1016/j.cvex.2009.01.007. PMID 19341950.
- Gaughan JB, Hogan LA, Wallage A (2015). Abstract: Thermoregulation in marsupials and monotremes, chapter of Marsupials and monotremes: nature's enigmatic mammals. Nova Science Publishers, Incorporated. ISBN 978-1-63483-487-2. Retrieved 20 April 2022.
- Anthes E (25 July 2024). "Rabies Is Spreading in South African Seals, Scientists Say". The New York Times -US. ISSN 0362-4331. Retrieved 4 August 2024.
- "Alert on rabies risk along the Western Cape coast: be cautious". Western Cape Government. Retrieved 4 August 2024.
- The Merck Manual (11th ed.). 1983. p. 183.
- The Merck manual of Medical Information (Second Home ed.). 2003. p. 484.
- Turton J (2000). "Rabies: a killer disease". National Department of Agriculture. Archived from the original on 23 September 2006.
- Jackson AC, Wunner WH (2002). Rabies. Academic Press. p. 290. ISBN 978-0-12-379077-4. Archived from the original on 8 January 2014.
- Lynn DJ, Newton HB, Rae-Grant AD (2012). The 5-Minute Neurology Consult. Lippincott Williams & Wilkins. pp. 414–. ISBN 978-1-4511-0012-9.
- Davis LE, King MK, Schultz JL (2005). Fundamentals of neurologic disease. Demos Medical Publishing. p. 73. ISBN 978-1-888799-84-2. Archived from the original on 8 January 2014.
- "Rabies FAQs: Exposure, prevention and treatment". RabiesAlliance.org. Archived from the original on 24 September 2016.
- Srinivasan A, Burton EC, Kuehnert MJ, Rupprecht C, Sutker WL, Ksiazek TG, et al. (March 2005). "Transmission of rabies virus from an organ donor to four transplant recipients". The New England Journal of Medicine. 352 (11): 1103–11. doi:10.1056/NEJMoa043018. PMID 15784663.
- "Human Rabies Prevention – United States, 2008 Recommendations of the Advisory Committee on Immunization Practices". Centers for Disease Control and Prevention. 23 May 2008. Retrieved 11 February 2020.
- Kahn CM, Line S, eds. (2010). The Merck Veterinary Manual (10th ed.). Kendallville, Indiana: Courier Kendallville, Inc. p. 1193. ISBN 978-0-911910-93-3.
- Dean DJ, Abelseth MK (1973). "Ch. 6: The fluorescent antibody test". In Kaplan MM, Koprowski H (eds.). Laboratory techniques in rabies. Monograph series. Vol. 23 (3rd ed.). World Health Organization. p. 73. ISBN 978-92-4-140023-7.
- ^ Fooks AR, Johnson N, Freuling CM, Wakeley PR, Banyard AC, McElhinney LM, et al. (September 2009). "Emerging technologies for the detection of rabies virus: challenges and hopes in the 21st century". PLOS Neglected Tropical Diseases. 3 (9): e530. doi:10.1371/journal.pntd.0000530. PMC 2745658. PMID 19787037.
- Tordo N, Bourhy H, Sacramento D (1994). "Ch. 10: PCR technology for lyssavirus diagnosis". In Clewley JP (ed.). The Polymerase Chain Reaction (PCR) for Human Viral Diagnosis. CRC Press. pp. 125–145. ISBN 978-0-8493-4833-4.
- David D, Yakobson B, Rotenberg D, Dveres N, Davidson I, Stram Y (June 2002). "Rabies virus detection by RT-PCR in decomposed naturally infected brains". Veterinary Microbiology. 87 (2): 111–18. doi:10.1016/s0378-1135(02)00041-x. PMID 12034539.
- Biswal M, Ratho R, Mishra B (September 2007). "Usefulness of reverse transcriptase-polymerase chain reaction for detection of rabies RNA in archival samples". Japanese Journal of Infectious Diseases. 60 (5): 298–99. doi:10.7883/yoken.JJID.2007.298. PMID 17881871.
- ^ Ly S, Buchy P, Heng NY, Ong S, Chhor N, Bourhy H, Vong S (September 2009). Carabin H (ed.). "Rabies situation in Cambodia". PLOS Neglected Tropical Diseases. 3 (9): e511. doi:10.1371/journal.pntd.0000511. PMC 2731168. PMID 19907631. e511.
- Dürr S, Naïssengar S, Mindekem R, Diguimbye C, Niezgoda M, Kuzmin I, et al. (March 2008). Cleaveland S (ed.). "Rabies diagnosis for developing countries". PLOS Neglected Tropical Diseases. 2 (3): e206. doi:10.1371/journal.pntd.0000206. PMC 2268742. PMID 18365035. e206.
- ^ "New Rapid Rabies Test Could Revolutionize Testing and Treatment". CDC Online Newsroom. Centers for Disease Control and Prevention. 16 May 2018. Retrieved 23 May 2018.
- "Rabies: Differential Diagnoses & Workup". eMedicine Infectious Diseases. 3 October 2008. Archived from the original on 28 November 2010. Retrieved 30 January 2010.
- Taylor DH, Straw BE, Zimmerman JL, D'Allaire S (2006). Diseases of swine. Oxford: Blackwell. pp. 463–65. ISBN 978-0-8138-1703-3. Retrieved 30 January 2010.
- Minagar A, Alexander JS (2005). Inflammatory Disorders Of The Nervous System: Pathogenesis, Immunology, and Clinical Management. Humana Press. ISBN 978-1-58829-424-1.
- Geison GL (April 1978). "Pasteur's work on rabies: reexamining the ethical issues". The Hastings Center Report. 8 (2): 26–33. doi:10.2307/3560403. JSTOR 3560403. PMID 348641.
- Srivastava AK, Sardana V, Prasad K, Behari M (March 2004). "Diagnostic dilemma in flaccid paralysis following anti-rabies vaccine". Neurology India. 52 (1): 132–33. PMID 15069272. Archived from the original on 2 August 2009.
- Reece JF, Chawla SK (September 2006). "Control of rabies in Jaipur, India, by the sterilisation and vaccination of neighbourhood dogs". The Veterinary Record. 159 (12): 379–383. doi:10.1136/vr.159.12.379. PMID 16980523. S2CID 5959305.
- "Compendium of Animal Rabies Prevention and Control" (PDF). National Association of State Public Health Veterinarians. 31 December 2007. Archived from the original (PDF) on 12 July 2010. Retrieved 3 January 2010.
- 2007 Annual Report (PDF) (Report). Bureau of Communicable Disease Control and Prevention. 2007. Archived from the original (PDF) on 11 May 2020. Retrieved 8 March 2011.
- "World Rabies Day". World Health Organization (WHO). Archived from the original on 31 December 2011.
- Meltzer MI (October–December 1996). "Assessing the costs and benefits of an oral vaccine for raccoon rabies: a possible model". Emerging Infectious Diseases. 2 (4): 343–9. doi:10.3201/eid0204.960411. PMC 2639934. PMID 8969251.
- ^ Grambo RL (1995). The World of the Fox. Vancouver: Greystone Books. pp. 94–95. ISBN 978-0-87156-377-4.
- "Rabies in the U.S." Centers for Disease Control and Prevention (CDC). 22 April 2011. Archived from the original on 31 December 2011. Retrieved 31 December 2011.
- ^ Lite J (8 October 2008). "Medical Mystery: Only One Person Has Survived Rabies without Vaccine – But How?". Scientific American. Archived from the original on 5 November 2009. Retrieved 30 January 2010.
- "Human rabies: better coordination and emerging technology to improve access to vaccines". World Health Organization. Archived from the original on 24 February 2017. Retrieved 23 February 2017.
- "Use of a Reduced (4-Dose) Vaccine Schedule for Postexposure Prophylaxis to Prevent Human Rabie" (PDF). Centers for Disease Control and Prevention (CDC). Archived from the original (PDF) on 25 July 2011.
- "Cost of Rabies Prevention". U.S. Centers for Disease Control and Prevention. 11 June 2019. Archived from the original on 29 March 2016.
- "BNF via NICE is only available in the UK". The National Institute for Health and Care Excellence. UK.
- "Rabies immunoglobulin". Milton Keynes Formulary Formulary.
- "Rabies – Vaccination". National Health Services (NHS). UK. 23 October 2017.
- Park K (2013). Park's Textbook of Preventive and Social Medicine (22nd ed.). Banarsidas Bhanot, Jabalpur. p. 254. ISBN 978-93-82219-02-6.
- Hicks DJ, Fooks AR, Johnson N (September 2012). "Developments in rabies vaccines". Clinical and Experimental Immunology. 169 (3): 199–204. doi:10.1093/clinids/13.4.644. PMC 3444995. PMID 22861358.
- "Rabies". World Health Organization. Archived from the original on 15 February 2015. Retrieved 1 February 2015.
- "Rabies & Australian bat lyssavirus information sheet". Health.vic.gov.au. Archived from the original on 18 August 2011. Retrieved 30 January 2012.
- National Center for Disease Control (2014). "National Guidelines on Rabies Prophylaxis" (PDF). Archived from the original (PDF) on 5 September 2014. Retrieved 5 September 2014.
- De Serres G, Skowronski DM, Mimault P, Ouakki M, Maranda-Aubut R, Duval B (June 2009). "Bats in the bedroom, bats in the belfry: reanalysis of the rationale for rabies postexposure prophylaxis". Clinical Infectious Diseases. 48 (11): 1493–99. doi:10.1086/598998. PMID 19400689.
- Despond O, Tucci M, Decaluwe H, Grégoire MC, S Teitelbaum J, Turgeon N (March 2002). "Rabies in a nine-year-old child: The myth of the bite". The Canadian Journal of Infectious Diseases. 13 (2): 121–25. doi:10.1155/2002/475909. PMC 2094861. PMID 18159381.
- Jackson AC (2020). "Chapter 17: Therapy of human rabies". In Jackson AC, Fooks AR (eds.). Rabies – Scientific Basis of the Disease and Its Management (4th ed.). Elsevier Science. p. 561. doi:10.1016/B978-0-12-818705-0.00017-0. ISBN 978-0-12-818705-0. S2CID 240895449.
- ^ Jackson AC (2016). "Human Rabies: a 2016 Update". Curr Infect Dis Rep (Review). 18 (11): 38. doi:10.1007/s11908-016-0540-y. PMID 27730539. S2CID 25702043.
- Jordan Lite (8 October 2008). "Medical Mystery: Only One Person Has Survived Rabies without Vaccine – But How?". Scientific American. Retrieved 16 October 2008.
- Willoughby RE (April 2007). "A cure for a rabies?". Sci Am. 296 (4): 88–95. Bibcode:2007SciAm.296d..88W. doi:10.1038/scientificamerican0407-88. PMID 17479635.
- Razner S (16 September 2019). "15 years after she survived rabies, Jeanna Giese seeks to save others from it". FDL Reporter. Retrieved 29 September 2024.
- Zeiler FA, Jackson AC (2016). "Critical Appraisal of the Milwaukee Protocol for Rabies: This Failed Approach Should Be Abandoned". Can J Neurol Sci (Review). 43 (1): 44–51. doi:10.1017/cjn.2015.331. PMID 26639059.
- Appolinario CM, Jackson AC (January 2015). "Antiviral therapy for human rabies". Antiviral Therapy. 20 (1): 1–10. doi:10.3851/IMP2851. hdl:11449/158463. PMID 25156675. Retrieved 14 November 2024.
- ^ "Rabies". World Health Organization (WHO). September 2011. Archived from the original on 31 December 2011. Retrieved 31 December 2011.
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–2128. doi:10.1016/S0140-6736(12)61728-0. hdl:10536/DRO/DU:30050819. PMC 10790329. PMID 23245604. S2CID 1541253.
- Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. (April 2015). "Estimating the global burden of endemic canine rabies". PLOS Neglected Tropical Diseases. 9 (4): e0003709. doi:10.1371/journal.pntd.0003709. PMC 4400070. PMID 25881058.
- "Rabies". World Health Organization. Archived from the original on 15 February 2017. Retrieved 23 February 2017.
- Dugan E (30 April 2008). "Dead as a dodo? Why scientists fear for the future of the Asian vulture". The Independent. London. Archived from the original on 17 May 2008. Retrieved 11 October 2008.
India now has the highest rate of human rabies in the world.
- ^ Harris G (6 August 2012). "Where Streets Are Thronged With Strays Baring Fangs". New York Times. Archived from the original on 8 August 2012. Retrieved 6 August 2012.
- "Medicine challenges Indian superstition". DW.DE. 31 December 2012. Archived from the original on 31 January 2013.
- "Essential rabies maps". World Health Organization (WHO). Archived from the original on 17 February 2010.
- "CDC – Rabies Surveillance in the U.S." www.cdc.gov. Archived from the original on 18 January 2017. Retrieved 10 April 2017.
- Fox M (7 September 2007). "U.S. free of canine rabies virus". Reuters. Archived from the original on 17 May 2017. Retrieved 11 April 2017.
"We don't want to misconstrue that rabies has been eliminated – dog rabies virus has been," CDC rabies expert Dr. Charles Rupprecht told Reuters in a telephone interview.
- ^ Pieracci EG, Pearson CM, Wallace RM, Blanton JD, Whitehouse ER, Ma X, et al. (June 2019). "Vital Signs: Trends in Human Rabies Deaths and Exposures – United States, 1938–2018". MMWR. Morbidity and Mortality Weekly Report. 68 (23): 524–28. doi:10.15585/mmwr.mm6823e1. PMC 6613553. PMID 31194721.
- Blackburn D (2022). "Human Rabies – Texas, 2021". MMWR. Morbidity and Mortality Weekly Report. 71 (49): 1547–49. doi:10.15585/mmwr.mm7149a2. PMC 9762899. PMID 36480462.
- "Surveillance Report - Annual Epidemiological Report for 2015 – Rabies" (PDF). European Centre for Disease Prevention and Control (ECDC). Retrieved 30 August 2018.
- "Switzerland ended rabies epidemic by air dropping vaccinated chicken heads from helicopters". thefactsource.com. 20 November 2019. Retrieved 10 December 2019.
- "Rabies in Africa: The RESOLAB network". 29 June 2015. Archived from the original on 3 August 2016. Retrieved 18 April 2016.
- "Ministero della Salute: "Italia è indenne dalla rabbia". l'Ultimo caso nel 2011 – Quotidiano Sanità". Archived from the original on 3 June 2016. Retrieved 18 April 2016.
- "Man dies from rabies after bat bite". BBC News. 24 November 2002.
- "Rabies". NHS. 23 February 2017. Retrieved 30 August 2018.
- "Q&A: Rabies". BBC News. 17 April 2015. Retrieved 30 August 2018.
- Mehnert E (1988). "Rabies och bekämpningsåtgärder i 1800-talets Sverige" [Rabies and remedies in 19th century Sweden]. Svensk veterinärtidning [Swedish veterinary magazine] (in Swedish). 1988 (40). Sveriges veterinärförbund: 277–288. OCLC 939018709.
- "Fladdermusrabies" [Bat rabies]. Statens Veterinärmedicinska Anstalt [State Veterinary Medical Institution] (in Swedish). Retrieved 28 October 2022.
- "Cómo México se convirtió en el primer país del mundo libre de rabia transmitida por perros" [How Mexico became the first country in the world free of rabies transmitted by dogs]. BBC News (in Spanish). 12 November 2019. Retrieved 12 November 2019.
- "Rabies in the South-East Asia Region". World Health Organization. 2024.
In the South-East Asia Region, rabies is endemic in Nepal, Sri Lanka and Thailand
- ^ Hicks R (3 November 2023). "Rabies is Spreading in South Asia Fueled by Inequality and Neglect". Eco‑Business.
- Abbas SS, Kakkar M (February 2015). "Rabies control in India: a need to close the gap between research and policy". Bull World Health Organ. 93 (2): 131–32. doi:10.2471/BLT.14.140723 (inactive 22 November 2024). PMC 4339964. PMID 25883407.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - Chowdhury FR, Basher A, Amin MR, Hassan N, Patwary MI (2015). "Rabies in South Asia: fighting for elimination". Recent Pat Anti-Infect Drug Discov. 10 (1): 30–34. doi:10.2174/1574891x10666150410130024. PMID 25858305.
- ^ Thanapongtharm W, Suwanpakdee S, Chumkaeo A, Gilbert M, Wiratsudakul A (December 2021). "Current characteristics of animal rabies cases in Thailand and relevant risk factors identified by a spatial modeling approach". PLOS Negl Trop Dis. 15 (12): e0009980. doi:10.1371/journal.pntd.0009980. PMC 8668119. PMID 34851953.
- CDC (2024). "Thailand". Travelers' health. Center for Disease Control and Prevention.
Rabid dogs are commonly found,rabies treatment is often available.&text=Since children are more likely,for children traveling to Thailand
- CDC (2024). "Thailand". Travelers' health. Center for Disease Control and Prevention.
Dogs infected with rabies are sometimes found in Thailand. If rabies exposures occur while in Thailand, rabies vaccines are typically available throughout most of the country.
- ^ Baron JN, Chevalier V, Ly S, Duong V, Dussart P, Fontenille D, Peng YS, Martínez-López B (June 2022). "Accessibility to rabies centers and human rabies post-exposure prophylaxis rates in Cambodia: A Bayesian spatio-temporal analysis to identify optimal locations for future centers". PLOS Negl Trop Dis. 16 (6): e0010494. doi:10.1371/journal.pntd.0010494. PMC 9491732. PMID 35771752.
- CDC (2024). "Cambodia". Travelers' Health. Center for Disease Control.
Dogs infected with rabies are commonly found in Cambodia. If rabies exposures occur while in Cambodia, rabies vaccines are typically available throughout most of the country.
- Adamson PB (1977). "The spread of rabies into Europe and the probable origin of this disease in antiquity". Journal of the Royal Asiatic Society of Great Britain & Ireland. Royal Asiatic Society of Great Britain and Ireland. 109 (2): 140–44. doi:10.1017/S0035869X00133829. JSTOR 25210880. PMID 11632333. S2CID 27354751.
- Dunlop RH, Williams DJ (1996). Veterinary Medicine: An Illustrated History. Mosby. ISBN 978-0-8016-3209-9.
- "Rabies: an ancient disease". Gobierno de México.
- Barrett AD, Stanberry LR (2009). Vaccines for Biodefense and Emerging and Neglected Diseases. Academic Press. p. 612. ISBN 978-0-08-091902-7. Archived from the original on 28 April 2016. Retrieved 8 January 2016.
- Baer GM (1991). The Natural History of Rabies (2nd ed.). CRC Press. ISBN 978-0-8493-6760-1.
The first major epizootic in North America was reported in 1768, continuing until 1771 when foxes and dogs carried the disease to swine and domestic animals. The malady was so unusual that it was reported as a new disease
- Than K (27 October 2010). "'Zombie Virus' Possible via Rabies-Flu Hybrid?". National Geographic. Archived from the original on 13 September 2015. Retrieved 13 September 2015.
- Santo Tomás Pérez M (2002). La asistencia a los enfermos en Castilla en la Baja Edad Media. Universidad de Valladolid. pp. 172–3. ISBN 84-688-3906-X – via Biblioteca Virtual Miguel de Cervantes.
- Ettinger SJ, Feldman EC (1995). Textbook of Veterinary Internal Medicine (4th ed.). W.B. Saunders Company. ISBN 978-0-7216-6795-9.
Further reading
- Pankhurst, Richard. "The history and traditional treatment of rabies in Ethiopia." Medical History 14, no. 4 (1970): 378–389.
External links
- "Rabies". Centers for Disease Control and Prevention. Retrieved 12 August 2012.
- "Rhabdoviridae". Virus Pathogen Database and Analysis Resource (ViPR).
- "OIE's Rabies Portal". Archived from the original on 13 August 2020.
- Karuturi S (20 June 2009). "Videos of Aerophobia and Hydrophobia in a suspected Rabies case". Doctors Hangout. Archived from the original on 2 April 2016.
- "Rabies virus". NCBI Taxonomy Browser. 11292.
| Classification | D |
|---|---|
| External resources |
| Rabies | |
|---|---|
| In popular culture | |
| Topics | |
| Viruses | |
| Outbreaks | Sarawak rabies outbreak |
| Countries | |
| Complications | |
| People | |
| Related articles | |
| Zoonotic viral diseases (A80–B34, 042–079) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arthropod -borne |
| ||||||||||||||||||||||||||||||||
| Mammal -borne |
| ||||||||||||||||||||||||||||||||
| Domestic cats | |||||
|---|---|---|---|---|---|
| Felinology | |||||
| Health | |||||
| Behavior | |||||
| Human–cat interaction | |||||
| Registries |
| ||||
| Breeds (full list) (experimental) |
| ||||
| Landraces | |||||
| Diseases and disorders |
| ||||
| Cats by country | |||||
| Related | |||||
| Dogs | |
|---|---|
| Types | |
| Breeds | |
| Roles | |
| Behavior | |
| Human–dog interaction | |
| Health | |
| Training | |
| Related | |