| |
| Names | |
|---|---|
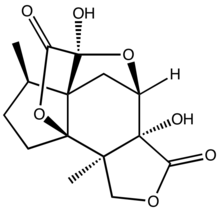
| IUPAC name (1R,2R,6R,7R,9S,10R,11R)-6,9-Dihydroxy-2,11-dimethyl-4,8,14-trioxapentacyclohexadecane-5,15-dione | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C15H18O7 |
| Molar mass | 310.302 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Jiadifenolide is a sesquiterpenoid natural product with neurotrophic activity, found in Illicium jiadifengpi. Its biological activity and congested polycyclic structure have made it a popular target for total synthesis.
Isolation and bioactivity
The seco-prezizaane-type sesquiterpenoid jiadifenolide was isolated in 2009 from the fruit of the flowering plant Illicium jiadifengpi. Chemical synthesis enabled preliminary assessment of its in vitro activity in promoting neurite outgrowth.
Chemical synthesis
Jiadifenolide has been the subject of synthetic study in several academic labs.
The first total synthesis, reported in 2009, employed an asymmetric Robinson annulation and a translactonization reaction to construct the core of the molecule. Two further syntheses, a chiral-pool approach from (+)-pulegone and a racemic synthesis relying on a samarium diiodide–mediated reductive cyclization, were reported almost simultaneously in 2014. A second chiral-pool synthesis (from (+)-citronellal) reported in 2015 shortened the synthetic sequence, with a double Michael addition as the key transformation of eight steps, allowing the synthesis of gram-scale quantities of jiadifenolide.
References
- Kubo, Miwa; Okada, Chihiro; Huang, Jian-Mei; Harada, Kenichi; Hioki, Hideaki; Fukuyama, Yoshiyasu (19 November 2009). "Novel Pentacyclic seco-Prezizaane-Type Sesquiterpenoids with Neurotrophic Properties from Illicium jiadifengpi". Organic Letters. 11 (22): 5190–5193. doi:10.1021/ol9021029. PMID 19873982.
- Trzoss, Lynnie; Xu, Jing; Lacoske, Michelle H.; Mobley, William C.; Theodorakis, Emmanuel A. (10 May 2013). "Illicium Sesquiterpenes: Divergent Synthetic Strategy and Neurotrophic Activity Studies". Chemistry: A European Journal. 19 (20): 6398–6408. doi:10.1002/chem.201300198. PMC 3875175. PMID 23526661.
- Downer-Riley, Nadale K.; Jackson, Yvette A. (2012). "Highlight syntheses". Annual Reports on the Progress of Chemistry, Section B. 108: 147. doi:10.1039/c2oc90006h.
- "(−)-Jiadifenolide". Chemistry World. Royal Society of Chemistry. Retrieved 29 July 2015.
- Xu, Jing; Trzoss, Lynnie; Chang, Weng K.; Theodorakis, Emmanuel A. (11 April 2011). "Enantioselective Total Synthesis of (−)-Jiadifenolide". Angewandte Chemie International Edition. 50 (16): 3672–3676. doi:10.1002/anie.201100313. PMC 3159889. PMID 21400650.
- Siler, David A.; Mighion, Jeffrey D.; Sorensen, Erik J. (19 May 2014). "An Enantiospecific Synthesis of Jiadifenolide". Angewandte Chemie International Edition. 53 (21): 5332–5335. doi:10.1002/anie.201402335. PMC 4153357. PMID 24757120.
- Paterson, Ian; Xuan, Mengyang; Dalby, Stephen M. (7 July 2014). "Total Synthesis of Jiadifenolide". Angewandte Chemie International Edition. 53 (28): 7286–7289. doi:10.1002/anie.201404224. PMC 4320761. PMID 24861364.
- Lu, Hai-Hua; Martinez, Michael D.; Shenvi, Ryan A. (15 June 2015). "An eight-step gram-scale synthesis of (−)-jiadifenolide". Nature Chemistry. 7 (7): 604–607. Bibcode:2015NatCh...7..604L. doi:10.1038/nchem.2283. PMID 26100810. S2CID 10715433.