This is a list of aminorex analogues. Aminorex itself is a stimulant drug with a 5-phenyl-2-amino-oxazoline structure. It was developed in the 1960s as an anorectic, but withdrawn from sale after it was discovered that extended use produced pulmonary hypertension, often followed by heart failure, which resulted in a number of deaths. A designer drug analogue 4-methylaminorex appeared on the illicit market in the late 1980s but did not attract significant popularity due to its steep dose-response curve and tendency to produce seizures. Pemoline, the 4-keto derivative of aminorex, had been discovered several years earlier, and derivatives of this type appeared to be effective stimulants with comparatively low toxicity. Pemoline was sold for around 25 years as a therapy for ADHD and relief of fatigue, before being withdrawn from the market in 2005 because of rare but serious cases of liver failure. More recently in around 2014 another derivative 4,4'-dimethylaminorex started to be sold illicitly, but again swiftly lost popularity due to a spate of fatal overdose cases. A number of related compounds are known, and new derivatives have continued to appear on the designer drug market.
List of substituted aminorex derivatives
| Structure | Common name | Chemical name | CAS number |
|---|---|---|---|
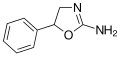
|
Aminorex | 5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 2207-50-3 |
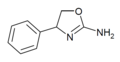
|
Rexamino | 4-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 52883-35-9 |

|
4'-Fluoroaminorex (4'-FAR) | 5-(4-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | 2967-77-3 |
| Clominorex | 5-(4-chlorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | 3876-10-6 | |

|
Fluminorex | 5--4,5-dihydro-1,3-oxazol-2-amine | 720-76-3 |

|
Methylenedioxyaminorex | 5-(3,4-methylenedioxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 3865-98-3 |

|
2C-B-aminorex (2C-B-AR) | 5-(2,5-dimethoxy-4-bromophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |

|
N,N-Dimethylaminorex (N,N-DMAR) | N,N-dimethyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 32968-41-5 |

|
Pemoline | 2-amino-5-phenyl-1,3-oxazol-4(5H)-one | 2152-34-3 |
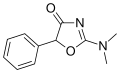
|
Thozalinone | 2-(dimethylamino)-5-phenyl-1,3-oxazol-4(5H)-one | 655-05-0 |

|
Fenozolone | 2-ethylamino-5-phenyl-1,3-oxazol-4-one | 15302-16-6 |

|
Cyclazodone | 2-(cyclopropylamino)-5-phenyl-1,3-oxazol-4-one | 14461-91-7 |
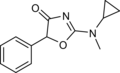
|
N-Methylcyclazodone | 2-(cyclopropyl(methyl)amino)-5-phenyl-1,3-oxazol-4-one | 14461-92-8 |

|
Ephedroxane | (4S,5R)-3,4-dimethyl-5-phenyl-1,3-oxazolidin-2-one | 16251-46-0 |

|
3-Methylaminorex | 3-methyl-5-phenyl-2-oxazolidinimine | 75343-73-6 |

|
4-Methylaminorex (4-MAR) | 4-methyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 3568-94-3 |

|
4-Ethylaminorex (4-EAR) | 4-ethyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | 1364933-63-0 |
|
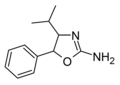
4-Isopropylaminorex | 4-isopropyl-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | |

|
4-Isobutylaminorex | 4-(2-methylpropyl)-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | |

|
4-tert-butylaminorex | 4-(1,1-dimethylethyl)-5-phenyl-4,5-dihydro-1,3-oxazol-2-amine | |

|
4,N-Dimethylaminorex (4,N-DMAR) | 4,5-dihydro-N,4-dimethyl-5-phenyl-2-oxazolamine | 2207-49-0 |

|
3,4-Dimethylaminorex (3,4-DMAR) | 3,4-dimethyl-5-phenyl-2-oxazolidinimine | 82485-31-2 |

|
4,4'-Dimethylaminorex (4,4'-DMAR) | 4-methyl-5-(4-methylphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445569-01-6 |

|
2'-Fluoro-4-methylaminorex (2F-MAR) | 4-methyl-5-(2-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |

|
3'-Fluoro-4-methylaminorex (3F-MAR) | 4-methyl-5-(3-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
|
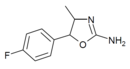
4'-Fluoro-4-methylaminorex (4F-MAR) | 4-methyl-5-(4-fluorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1364933-64-1 |

|
4'-Chloro-4-methylaminorex (4C-MAR) | 4-methyl-5-(4-chlorophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |
|
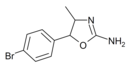
4'-Bromo-4-methylaminorex (4B-MAR) | 4-methyl-5-(4-bromophenyl)-4,5-dihydro-1,3-oxazol-2-amine | |

|
4'-Methoxy-4-methylaminorex (4'-MeO-4-MAR) | 4-methyl-5-(4-methoxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445570-65-9 |

|
3',4',5'-Trimethoxy-4-methylaminorex (TM-4-MAR) | 4-methyl-5-(3,4,5-trimethoxyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445571-92-5 |

|
3',4'-Methylenedioxy-4-methylaminorex (MDMAR) | 4-methyl-5-(3,4-methylenedioyphenyl)-4,5-dihydro-1,3-oxazol-2-amine | 1445573-16-9 |
See also
- Berefrine
- RO5166017
- List of methylphenidate analogues
- Substituted benzofuran
- Substituted cathinone
- Substituted phenylmorpholine
References
- Meschino JA, Poos GI. 2-amino-5,6-dihydro-4H-1,3-oxazines and a process for their preparation. US Patent 3115494, 1961
- Poos GI. 2-amino-5-aryloxazoline products. US Patent 3161650, 1962
- Poos GI, Carson JR, Rosenau JD, Roszkowski AP, Kelley NM, Mcgowin J (May 1963). "2-Amino-5-aryl-2-oxazolines. Potent New Anorectic Agents". Journal of Medicinal Chemistry. 6 (3): 266–272. doi:10.1021/jm00339a011. PMID 14185981.
- Gurtner HP (1985). "Aminorex and pulmonary hypertension. A review". Cor et Vasa. 27 (2–3): 160–171. PMID 3928246.
- Davis FT, Brewster ME (March 1988). "A fatality involving U4Euh, a cyclic derivative of phenylpropanolamine". Journal of Forensic Sciences. 33 (2): 549–553. doi:10.1520/JFS11971J. PMID 3373171.
- Bunker CF, Johnson M, Gibb JW, Bush LG, Hanson GR (May 1990). "Neurochemical effects of an acute treatment with 4-methylaminorex: a new stimulant of abuse". European Journal of Pharmacology. 180 (1): 103–111. doi:10.1016/0014-2999(90)90597-y. PMID 1973111.
- Gaine SP, Rubin LJ, Kmetzo JJ, Palevsky HI, Traill TA (November 2000). "Recreational use of aminorex and pulmonary hypertension". Chest. 118 (5): 1496–1497. doi:10.1378/chest.118.5.1496. PMID 11083709.
- Meririnne E, Kajos M, Kankaanpää A, Koistinen M, Kiianmaa K, Seppälä T (August 2005). "Rewarding properties of the stereoisomers of 4-methylaminorex: involvement of the dopamine system". Pharmacology, Biochemistry, and Behavior. 81 (4): 715–724. doi:10.1016/j.pbb.2005.04.020. PMID 15982727. S2CID 21142560.
- Schmidt L, Scheffler H. Central nervous system stimulant. US Patent 2892753, 1957
- "Hardy RA, Howell CF, Quinones NQ. Method of producing central nervous system stimulation and anorexia. US Patent 3313688, 1964". Archived from the original on 2021-05-31. Retrieved 2019-06-14.
- "Guidicelli DP, Najer H. 5-phenyl-2-cyclopropylamino-4-oxazolinone, and process for making the same. US Patent 3609159, 1967". Archived from the original on 2021-05-31. Retrieved 2019-06-14.
- Marotta PJ, Roberts EA (May 1998). "Pemoline hepatotoxicity in children". The Journal of Pediatrics. 132 (5): 894–897. doi:10.1016/s0022-3476(98)70329-4. PMID 9602211.
- Safer DJ, Zito JM, Gardner JE (June 2001). "Pemoline hepatotoxicity and postmarketing surveillance". Journal of the American Academy of Child and Adolescent Psychiatry. 40 (6): 622–629. doi:10.1097/00004583-200106000-00006. PMID 11392339.
- Etwel FA, Rieder MJ, Bend JR, Koren G (2008). "A surveillance method for the early identification of idiosyncratic adverse drug reactions". Drug Safety. 31 (2): 169–180. doi:10.2165/00002018-200831020-00006. PMID 18217792. S2CID 19964105.
- Shader RI (April 2017). "Risk Evaluation and Mitigation Strategies (REMS), Pemoline, and What Is a Signal?". Clinical Therapeutics. 39 (4): 665–669. doi:10.1016/j.clinthera.2017.03.008. PMID 28366595.
- Brandt SD, Baumann MH, Partilla JS, Kavanagh PV, Power JD, Talbot B, et al. (2014). "Characterization of a novel and potentially lethal designer drug (±)-cis-para-methyl-4-methylaminorex (4,4'-DMAR, or 'Serotoni')". Drug Testing and Analysis. 6 (7–8): 684–695. doi:10.1002/dta.1668. PMC 4128571. PMID 24841869.
- Coppola M, Mondola R (July 2015). "4,4'-DMAR: chemistry, pharmacology and toxicology of a new synthetic stimulant of abuse". Basic & Clinical Pharmacology & Toxicology. 117 (1): 26–30. doi:10.1111/bcpt.12399. PMID 25819702.
- Maier J, Mayer FP, Luethi D, Holy M, Jäntsch K, Reither H, et al. (August 2018). "The psychostimulant (±)-cis-4,4'-dimethylaminorex (4,4'-DMAR) interacts with human plasmalemmal and vesicular monoamine transporters". Neuropharmacology. 138: 282–291. doi:10.1016/j.neuropharm.2018.06.018. PMID 29908239. S2CID 49274224.
- Russell BR, Beresford RA, Schmierer DM, McNaughton N, Clark CR (1995). "Stimulus properties of some analogues of 4-methylaminorex". Pharmacology, Biochemistry, and Behavior. 51 (2–3): 375–378. doi:10.1016/0091-3057(94)00407-a. PMID 7667356. S2CID 28367828.
- Zheng Y, Russell B, Schmierer D, Laverty R (January 1997). "The effects of aminorex and related compounds on brain monoamines and metabolites in CBA mice". The Journal of Pharmacy and Pharmacology. 49 (1): 89–96. doi:10.1111/j.2042-7158.1997.tb06758.x. PMID 9120777. S2CID 20224300.
- McLaughlin G, Morris N, Kavanagh PV, Power JD, Twamley B, O'Brien J, et al. (July 2015). "Synthesis, characterization, and monoamine transporter activity of the new psychoactive substance 3',4'-methylenedioxy-4-methylaminorex (MDMAR)". Drug Testing and Analysis. 7 (7): 555–564. doi:10.1002/dta.1732. PMC 5331736. PMID 25331619.
- Maier J, Mayer FP, Brandt SD, Sitte HH (October 2018). "DARK Classics in Chemical Neuroscience: Aminorex Analogues". ACS Chemical Neuroscience. 9 (10): 2484–2502. doi:10.1021/acschemneuro.8b00415. PMC 6287711. PMID 30269490.
- Fabregat-Safont D, Carbón X, Ventura M, Fornís I, Hernández F, Ibáñez M (June 2019). "Characterization of a recently detected halogenated aminorex derivative: para-fluoro-4-methylaminorex (4'F-4-MAR)". Scientific Reports. 9 (1): 8314. Bibcode:2019NatSR...9.8314F. doi:10.1038/s41598-019-44830-y. PMC 6549166. PMID 31165778.
- Rickli A, Kolaczynska K, Hoener MC, Liechti ME (May 2019). "Pharmacological characterization of the aminorex analogs 4-MAR, 4,4'-DMAR, and 3,4-DMAR". Neurotoxicology. 72: 95–100. Bibcode:2019NeuTx..72...95R. doi:10.1016/j.neuro.2019.02.011. PMID 30776375. S2CID 73474963.
- Hikino H, Ogata K, Kasahara Y, Konno C (May 1985). "Pharmacology of ephedroxanes". Journal of Ethnopharmacology. 13 (2): 175–191. doi:10.1016/0378-8741(85)90005-4. PMID 4021515.