 | |
| Clinical data | |
|---|---|
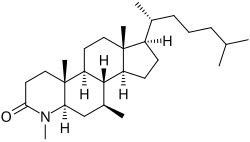
| Other names | L-733692; 4,7β-Dimethyl-4-aza-5α-cholestan-3-one |
| Routes of administration | By mouth |
| Drug class | 5α-Reductase inhibitor |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H49NO |
| Molar mass | 415.706 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
MK-386, also known as 4,7β-dimethyl-4-aza-5α-cholestan-3-one, is a synthetic, steroidal 5α-reductase inhibitor which was first reported in 1994 and was never marketed. It is a 4-azasteroid and a potent and selective inhibitor of 5α-reductase type I and shows high selectivity for inhibition of human 5α-reductase type I over 5α-reductase type II, with IC50 values of 0.9 nM and 154 nM, respectively. The drug was under investigation for potential treatment of androgen-dependent conditions such as acne and pattern hair loss (androgenic alopecia or baldness), but was discontinued in early clinical trials due to observations of hepatotoxicity such as elevated liver enzymes.
MK-386 has been found to decrease circulating concentrations of dihydrotestosterone (DHT) in men by 20 to 30%, which is in accordance with the fact that 5α-reductase type II is responsible for 70 to 80% of DHT production while 5α-reductase type I is responsible for 20 to 30%. In contrast to MK-386, the selective 5α-reductase type II inhibitor finasteride has been found to decrease DHT levels by about 70%, while the non-selective 5α-reductase inhibitor dutasteride decreases DHT levels by up to 98%. Co-administration of MK-386 and finasteride was found to produce near-complete (~95%) suppression of circulating DHT levels.
MK-386 has been found to significantly decrease concentrations of DHT in sebum, similarly to the selective 5α-reductase type II inhibitor finasteride. However, whereas finasteride results in only a modest reduction in sebum DHT levels of 15%, MK-386 has been found to produce a significantly greater reduction of 55%. While finasteride decreases semen DHT levels by approximately 88%, MK-386 has been found to have no effect on levels of DHT in semen. These findings are in accordance with the known tissue distribution of 5α-reductase isoforms.
MK-386 was assessed in the treatment of acne but failed to separate from placebo in effectiveness and was significantly inferior to antibiotic therapy with minocycline. In addition, the addition of MK-386 to minocycline failed to increase effectiveness relative to minocycline alone. A study of MK-386 treatment for one year in stumptail macaques found that the drug failed to increase scalp hair weight in a model of androgenic alopecia, in contrast to finasteride.
References
- ^ Bakshi RK, Patel GF, Rasmusson GH, Baginsky WF, Cimis G, Ellsworth K, et al. (November 1994). "4,7 beta-Dimethyl-4-azacholestan-3-one (MK-386) and related 4-azasteroids as selective inhibitors of human type 1 5 alpha-reductase". Journal of Medicinal Chemistry. 37 (23): 3871–3874. doi:10.1021/jm00049a003. PMID 7966146.
- ^ Ellsworth K, Azzolina B, Baginsky W, Bull H, Chang B, Cimis G, et al. (July 1996). "MK386: a potent, selective inhibitor of the human type 1 5alpha-reductase". The Journal of Steroid Biochemistry and Molecular Biology. 58 (4): 377–384. doi:10.1016/0960-0760(96)00050-7. PMID 8903421. S2CID 54344877.
- Frye SV (February 1996). "Inhibitors of 5-alpha-reductase". Current Pharmaceutical Design. 2 (1). Bentham Science Publishers: 59-84 (67). doi:10.2174/1381612802666220920215559. S2CID 252491829.
- Machetti F, Guarna A (2005). "Novel inhibitors of 5α-reductase". Expert Opinion on Therapeutic Patents. 12 (2): 201–215. doi:10.1517/13543776.12.2.201. ISSN 1354-3776. S2CID 85073794.
- Schwartz JI, Van Hecken A, De Schepper PJ, De Lepeleire I, Lasseter KC, Shamblen EC, et al. (August 1996). "Effect of MK-386, a novel inhibitor of type 1 5 alpha-reductase, alone and in combination with finasteride, on serum dihydrotestosterone concentrations in men". The Journal of Clinical Endocrinology and Metabolism. 81 (8): 2942–2947. doi:10.1210/jcem.81.8.8768856. PMID 8768856.
- Marchetti PM, Barth JH (March 2013). "Clinical biochemistry of dihydrotestosterone". Annals of Clinical Biochemistry. 50 (Pt 2): 95–107. doi:10.1258/acb.2012.012159. PMID 23431485. S2CID 8325257.
- Hoffman J, Sommer A (30 January 2007). "Anti-hormome Therapy: Principles of Endocrine Therapy of Cancer". In Bradbury R (ed.). Cancer. Springer Science & Business Media. pp. 49–. ISBN 978-3-540-33120-9.
- Kaufman KD (1 April 2004). "Clinical use of 5alpha-reductase inhibitors". In Nieschlag E, Behre HM (eds.). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 586–. ISBN 978-1-139-45221-2.
- ^ Schwartz JI, Tanaka WK, Wang DZ, Ebel DL, Geissler LA, Dallob A, et al. (May 1997). "MK-386, an inhibitor of 5alpha-reductase type 1, reduces dihydrotestosterone concentrations in serum and sebum without affecting dihydrotestosterone concentrations in semen". The Journal of Clinical Endocrinology and Metabolism. 82 (5): 1373–1377. doi:10.1210/jcem.82.5.3912. PMID 9141518.
- McConnell JD, Stoner E (18 April 2001). "5 alpha-Reductase inhibitors". Drug Discovery and Design. Advances in Protein Chemistry. 56. Academic Press: 143–180 (172). doi:10.1016/s0065-3233(01)56005-2. ISBN 978-0-08-049338-1. PMID 11329853.
- ^ Leyden J, Bergfeld W, Drake L, Dunlap F, Goldman MP, Gottlieb AB, et al. (March 2004). "A systemic type I 5 alpha-reductase inhibitor is ineffective in the treatment of acne vulgaris". Journal of the American Academy of Dermatology. 50 (3): 443–447. doi:10.1016/j.jaad.2003.07.021. PMID 14988688.
- ^ Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Advances in Urology. 2012: 530121. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
- Kaufman KD (December 2002). "Androgens and alopecia". Molecular and Cellular Endocrinology. 198 (1–2): 89–95. doi:10.1016/S0303-7207(02)00372-6. PMID 12573818. S2CID 2461147.
- Kaufman KD (2001). "5α-Reductase Inhibitors in the Treatment of Androgenetic Alopecia". International Journal of Cosmetic Surgery and Aesthetic Dermatology. 3 (2): 107–119. doi:10.1089/153082001753231036. ISSN 1530-8200.