| |
| Names | |
|---|---|
| Other names Maytansin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.047.944 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
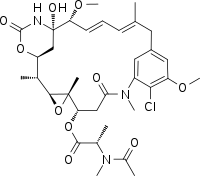
| Chemical formula | C34H46ClN3O10 |
| Molar mass | 692.20 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Maitansine (INN), or maytansine (USAN), is a cytotoxic agent. It inhibits the assembly of microtubules by binding to tubulin at the rhizoxin binding site. The maytansine binding site and binding mode has been characterized.
It is a macrolide of the ansamycin type and can be isolated from plants of the genus Maytenus.
Maytansinoids
Derivatives of maitansine are known as maytansinoids. Some are being investigated as the cytotoxic component of antibody-drug conjugates for cancer treatment, and the antibody-drug conjugate trastuzumab emtansine is an approved drug for the treatment of certain kinds of breast cancer in the EU and in the US.
Examples of maytansinoids are:
- Ansamitocin
- Mertansine / emtansine (DM1)
- Ravtansine / soravtansine (DM4)
See also
- ImmunoGen, developer of maytansinoid based drugs
References
- ^ National Cancer Institute: Definition of Maytansine
- Menchon, Grégory; Prota, Andrea E.; Lucena-Agell, Daniel; Bucher, Pascal; Jansen, Rolf; Irschik, Herbert; Müller, Rolf; Paterson, Ian; Díaz, J. Fernando; Altmann, Karl-Heinz; Steinmetz, Michel O. (2018-05-29). "A fluorescence anisotropy assay to discover and characterize ligands targeting the maytansine site of tubulin". Nature Communications. 9 (1). Springer Science and Business Media LLC. doi:10.1038/s41467-018-04535-8. ISSN 2041-1723. PMC 5974090.
- ^ Yu, T.-W.; Bai, L; Clade, D; Hoffmann, D; Toelzer, S; Trinh, KQ; Xu, J; Moss, SJ; Leistner, E (2002). "The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnemapretiosum". Proceedings of the National Academy of Sciences. 99 (12): 7968–7973. Bibcode:2002PNAS...99.7968Y. doi:10.1073/pnas.092697199. PMC 123004. PMID 12060743.
- Lopus, M; Oroudjev, E; Wilson, L; Wilhelm, S; Widdison, W; Chari, R; Jordan, MA (2010). "Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules". Mol Cancer Ther. 9 (10): 2689–99. doi:10.1158/1535-7163.MCT-10-0644. PMC 2954514. PMID 20937594.
- Chari, RV; Martell, BA; Gross, JL; et al. (January 1992). "Immunoconjugates containing novel maytansinoids: promising anticancer drugs" (PDF). Cancer Res. 52 (1): 127–31. PMID 1727373.
- "Kadcyla EPAR". European Medicines Agency (EMA). 17 September 2018.
- "Drug Approval Package: ado-trastuzumab emtansine". U.S. Food and Drug Administration (FDA). 22 February 2013. Archived from the original on 4 December 2019. Retrieved 3 December 2019.
This antineoplastic or immunomodulatory drug article is a stub. You can help Misplaced Pages by expanding it. |