Medical condition
| Gynecomastia | |
|---|---|
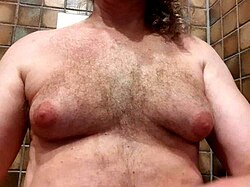 | |
| Adult male with significant gynecomastia | |
| Pronunciation | |
| Specialty | Endocrinology, plastic surgery |
| Complications | Psychological distress |
| Usual onset | Any age |
| Duration | Usually up to 2 years, but can be lifelong |
| Causes | Increased estrogen/androgen ratio (physiologic, medications, chronic kidney disease, obesity, cirrhosis, malnutrition, certain cancers, anabolic steroids) |
| Diagnostic method | physical exam, mammography (if indicated) |
| Differential diagnosis | Pseudogynecomastia, breast cancer |
| Treatment | Lifestyle modifications, aromatase inhibitors, SERMs, or surgery |
| Frequency | ~35% of men, up to 70% of adolescent boys |
Gynecomastia (also spelled gynaecomastia) is the non-cancerous enlargement of one or both breasts in men due to the growth of breast tissue as a result of a hormone imbalance between estrogens and androgens. Physically speaking, gynecomastia is completely benign, but it is associated with significant psychological distress, social stigma, and dysphoria.
Gynecomastia can be normal in newborn male babies due to exposure to estrogen from the mother, in adolescent boys going through puberty, in older men over the age of 50, and in obese men. Most occurrences of gynecomastia do not require diagnostic tests. Gynecomastia may be caused by abnormal hormone changes, any condition that leads to an increase in the ratio of estrogens/androgens such as liver disease, kidney failure, thyroid disease and some non-breast tumors. Alcohol and some drugs can also cause breast enlargement. Other causes may include Klinefelter syndrome, metabolic dysfunction, or a natural decline in testosterone production. This may occur even if the levels of estrogens and androgens are both appropriate, but the ratio is altered.
Gynecomastia is the most common benign disorder of the male breast tissue and affects 35% of men, being most prevalent between the ages of 50 and 69. It is normal for up to 70% of adolescent boys to develop gynecomastia to some degree. Of these, 75% resolve within two years of onset without treatment. If the condition does not resolve within 2 years, or if it causes embarrassment, pain or tenderness, treatment is warranted. Medical treatment of gynecomastia that has persisted beyond two years is often ineffective. Gynecomastia is different from "pseudogynecomastia", which is commonly present in men with obesity.
Medications such as aromatase inhibitors have been found to be effective and even in rare cases of gynecomastia from disorders such as aromatase excess syndrome or Peutz–Jeghers syndrome, but surgical removal of the excess tissue can be needed to correct the condition. In 2019, 24,123 male patients underwent the procedure in the United States, accounting for a 19% increase since 2000.
Definition
Gynecomastia is the abnormal non-cancerous enlargement of one or both breasts in men due to the growth of breast tissue as a result of a hormone imbalance between estrogen and androgen. Gynecomastia is different from "pseudogynecomastia", which is defined as an excess of skin and/or adipose tissue in the male breasts without the growth of true glandular breast tissue; this is commonly associated with obesity and can be ruled out by physical exam.
Signs and symptoms
In gynecomastia there is always enlargement of one or both breasts, symmetrically or asymmetrically, in a man. A soft, compressible, and mobile mass of breast tissue is felt under the nipple and its surrounding skin in contrast to softer fatty tissue which is not associated with a mass. It may also be accompanied by breast tenderness or nipple sensitivity, which is commonly associated with gynecomastia observed in adolescents, typically early in development. Gynecomastia that is painful, bothersome, rapidly-growing, associated with masses in other areas of the body, or persistent should be evaluated by a clinician for potential causes. Dimpling of the skin, nipple discharge, and nipple retraction are not typical features of gynecomastia and may be associated with other disorders. Milky discharge from the nipple is not a typical finding, but may be seen in a gynecomastic individual with a prolactin secreting tumor. An increase in the diameter of the areola and asymmetry of the chest are other possible signs of gynecomastia.
Much of the research on gynecomastia has focused on its causes and treatment, but little has explored its effects on mental health and overall quality of life. Gynecomastia has psychosocial implications that may be particularly challenging for adolescents who are experiencing physical maturation and self-identity formation, which includes body image disturbances, negative attitudes towards eating, self-esteem problems, social withdrawal, anxiety, and shame. Men with gynecomastia may appear anxious or stressed due to concerns about its appearance and the possibility of having breast cancer. Particular studies suggest that gynecomastia can lead to various psychological and social challenges, such as depression, anxiety and disordered eating.
Causes
Gynecomastia is thought to be caused by an altered ratio of estrogens to androgens mediated by an increase in estrogen action, a decrease in androgen action, or a combination of these two factors. Estrogen and androgens have opposing actions on breast tissue: estrogens stimulate proliferation while androgens inhibit proliferation. The cause of gynecomastia is unknown in around 25% of cases. Known causes can be physiologic (occurring normally) or non-physiologic due to underlying pathologies such as drug use, chronic disease, tumors, or malnutrition.
Physiologic
Physiologic or normal gynecomastia can occur at three timepoints in life: shortly after birth in both female and male infants, during puberty in adolescent males, and in older adults over the age of 60.
Newborns
60-90% of male and female newborns may show breast development at birth or in the first weeks of life. During pregnancy, the placenta converts the androgenic hormones dehydroepiandrosterone (DHEA) and DHEA sulfate to the estrogenic hormones estrone and estradiol, respectively; after these estrogens are produced by the placenta, they are transferred into the baby's circulation, thereby leading to temporary gynecomastia in the baby. In some infants, neonatal milk (also known as "witch's milk") can leak from the nipples. The temporary gynecomastia seen in newborn babies usually resolves after two or three weeks.
Adolescents

Hormonal imbalance (elevated ratio of estrogen to androgen) during early puberty, either due to decreased androgen production from the adrenals and/or increased conversion of androgens to estrogens, leads to transient gynecomastia in adolescent males. It can occur in up to 65% of adolescents as early as age 10 and peaks at ages 13 and 14. It is self-limited in 75–90% of adolescents and usually resolves spontaneously within 1 to 3 years as pubertal progression increases testosterone levels and cause regression of breast tissue. By age 17, only 10% of adolescent males have persistent gynecomastia.
Older adults
Declining testosterone levels and an increase in the level of subcutaneous fatty tissue seen as part of the normal aging process can lead to gynecomastia in older males. Increased fatty tissue, a major site of aromatase activity, leads to increased conversion of androgenic hormones such as testosterone to estrogens. Additionally, levels of sex hormone binding globulin (SHBG) increase with age and bind with less affinity to estrogen than androgens. Put together, the elevated ratio of estrogen to androgen leads to gynecomastia, also known as senile gynecomastia in this group. There is a 24–65% prevalence of senile gynecomastia in older males.
Non-physiologic
Drugs

About 10–25% of gynecomastia cases are estimated to result from the use of medications or exogenous chemicals. Drugs can increase estrogen activity or increase the estrogen to androgen ratio through various mechanisms, such as binding to estrogen receptors, promoting estrogen synthesis, providing precursors that can be aromatized into estrogen, causing damage to the testes, inhibiting testosterone synthesis, inhibiting the action of androgens, or displacing estrogen from SHBG. Drugs with good evidence for association with gynecomastia include cimetidine, ketoconazole, gonadotropin-releasing hormone analogues, human growth hormone, human chorionic gonadotropin, 5α-reductase inhibitors such as finasteride and dutasteride, certain estrogens used for prostate cancer, and antiandrogens such as bicalutamide, flutamide, and spironolactone.
Drugs with fair evidence for association with gynecomastia include calcium channel blockers such as verapamil, amlodipine, and nifedipine; risperidone, olanzapine, anabolic steroids, alcohol, opioids, efavirenz, alkylating agents, and omeprazole. Certain components of personal skin care products such as lavender or tea tree oil have been reported to cause prepubertal gynecomastia due to its estrogenic and anti-androgenic effects. Certain dietary supplements such as dong quai and Tribulus terrestris have also been associated with gynecomastia.
Refeeding gynecomastia
Malnutrition and significant loss of body fat suppress gonadotropin secretion, leading to hypogonadism. This is reversible when adequate nutrition resumes, where the return of gonadotropin secretion and gonadal function cause a transient imbalance of estrogen and androgen that mimics puberty, resulting in transient gynecomastia. This phenomenon, also known as refeeding gynecomastia, was first observed when men returning home from prison camps during World War II developed gynecomastia after resuming a normal diet. Similar to pubertal gynecomastia, refeeding gynecomastia resolves on its own in 1–2 years.
Chronic disease
Many kidney failure patients experience a hormonal imbalance due to the suppression of testosterone production and testicular damage from high levels of urea also known as uremia-associated hypogonadism. Additionally, gynecomastia has been observed in 50% of patients with chronic kidney disease undergoing dialysis. Similar to the mechanism behind refeeding gynecomastia, dialysis allows patients with renal failure who were previously malnourished to expand their diets and regain weight. Dialysis-associated gynecomastia resolves spontaneously within 1–2 years.
In individuals with liver failure or cirrhosis, the liver's ability to properly metabolize hormones such as estrogen may be impaired. Additionally, those with alcoholic liver disease are further put at risk for development of gynecomastia; ethanol may directly disrupt the synthesis of testosterone and the presence of phytoestrogens in alcoholic drinks may also contribute to a higher estrogen to testosterone ratio. Conditions that can cause malabsorption such as cystic fibrosis or ulcerative colitis may also produce gynecomastia.
A small proportion of male gynecomastia cases may be seen with rare inherited disorders such as spinal and bulbar muscular atrophy and the very rare aromatase excess syndrome.
Hypogonadism
Gynecomastia can be caused by absolute deficiency in androgen production due to primary or secondary hypogonadism. Primary hypogonadism results when there is damage to the testes (due to radiation, chemotherapy, infections, trauma, etc), leading to impaired androgen production. It can also be caused by chromosomal abnormality seen in Klinefelter syndrome, which is associated with gynecomastia in about 80% of cases. Secondary hypogonadism results when there is damage to the hypothalamus or pituitary (due to radiation, chemotherapy, infection, trauma, etc), and similarly lead to impaired androgen production. The net effect is reduced androgen production while serum estrogen levels (from peripheral aromatization of androgens) remain unaffected. The lack of androgen-mediated inhibition of breast tissue proliferation combined with relative estrogen excess result in gynecomastia.
Tumors
Testicular tumors such as Leydig cell tumors, Sertoli cell tumors (such as in Peutz–Jeghers syndrome) and hCG-secreting choriocarcinoma may result in rapid-onset gynecomastia by causing excess production of estrogen. Other tumors such as adrenal tumors, pituitary gland tumors (such as a prolactinoma), or lung cancer, can produce hormones that alter the male–female hormone balance and cause gynecomastia.
Individuals with prostate cancer who are treated with androgen deprivation therapy may experience gynecomastia.
Pathophysiology


The causes of common gynecomastia remain uncertain, but are thought to result from an imbalance between the actions of estrogen, which stimulates breast tissue growth, and androgens, which inhibit breast tissue growth. Breast prominence can result from enlargement of glandular breast tissue, chest adipose tissue (fat) and skin, and is typically a combination. As in females, estrogen stimulates the growth of breast tissue in males. In addition to directly stimulating breast tissue growth, estrogens indirectly decrease secretion of testosterone by suppressing luteinizing hormone secretion, resulting in decreased testicular secretion of testosterone.
Estrogen excess
One of the main mechanisms for imbalance between estrogens and androgens is the overproduction of estrogens. A possible cause may be a neoplasm that originates from estrogen-secreting cells. Tumors that produce hCG stimulate production of estradiol while reducing other testicular hormone production. Obesity is another common cause of excess serum estrogens due to the presence of aromatase in peripheral tissue, which is a protein that converts androgens into estrogens. Peutz-Jeghers syndrome is a rare cause of testicular tumors that affect aromatase expression, which results in elevated serum estrogen levels. Aromatase excess syndrome is a rare genetic disorder that leads to increased conversion of androgens to estrogens in the body.
Androgen deficiency
Primary hypogonadism (indicating an intrinsic problem with the testes in males) leads to decreased testosterone synthesis and increased conversion of testosterone to estradiol potentially leading to a gynecomastic appearance. Klinefelter syndrome is a notable example of a disorder that causes hypogonadism and gynecomastia, and has a higher risk of breast cancer in males (20–50 times higher than males without the disorder). Secondary hypogonadism (indicating a problem with the brain) leads to decreased production and release of luteinizing hormone (LH, a stimulatory signal for endogenous steroid hormone synthesis) which leads to decreased production of testosterone and estradiol in the testes.
Increased levels of sex hormone-binding globulin
Estrogens can increase blood levels of the protein sex hormone-binding globulin (SHBG), which binds free testosterone (the active form) more strongly than estrogen, leading to decreased action of testosterone in male breast tissue. Conditions such as hyperthyroidism and chronic liver disease affect levels of SHBG, leading to symptomatic gynecomastia.
Androgen resistance
Dysfunction in the androgen receptor prevents the effects of testosterone from acting on its target tissues. Androgen insensitivity syndromes result from the different degrees of resistance to the effects of androgens, and can cause external genitalia that may not be aligned with the genotype of the individual's sex chromosomes. Complete androgen insensitivity syndrome results in the failure to develop external genitalia such as the penis and scrotum along with development of breasts in an individual with testes. Partial androgen insensitivity syndrome may result in a variety of presentations. Minimal androgen insensitivity syndrome may present as gynecomastia in adolescence and may additionally be associated with infertility.
Medications
Medications are known to cause gynecomastia through several different mechanisms. These mechanisms include increasing estrogen levels, mimicking estrogen, decreasing levels of testosterone or other androgens, blocking androgen receptors, increasing prolactin levels, or through unidentified means. Potential causative agents include oral contraceptive pills, spironolactone, and anabolic steroids.
High levels of prolactin in the blood (which may occur as a result of certain tumors or as a side effect of certain medications) has been associated with gynecomastia. A high level of prolactin in the blood can inhibit the release of gonadotropin-releasing hormone and therefore cause secondary hypogonadism. Receptors for prolactin and other hormones including insulin-like growth factor 1, insulin-like growth factor 2, luteinizing hormone, progesterone, and human chorionic gonadotropin have been found in male breast tissue, but the impact of these various hormones on gynecomastia development is not well understood.
Chronic disease
Individuals who have cirrhosis or chronic liver disease may develop gynecomastia for several reasons. Those diagnosed with cirrhosis tend to have increased secretion of the androgenic hormone androstenedione from the adrenal glands, increased conversion of this hormone into various types of estrogen, and increased levels of SHBG, which leads to decreased blood levels of free testosterone. Around 10–40% of males with Graves' disease (a common form of hyperthyroidism) experience gynecomastia. Increased conversion of testosterone to estrogen by increased aromatase activity, increased levels of SHBG and increased production of testosterone and estradiol by the testes due to elevated levels of LH cause the gynecomastia. Proper treatment of the hyperthyroidism can lead to the resolution of the gynecomastia.
| Estrogen excess | Androgen deficiency | Increased levels of sex hormone-binding globulin | Androgen resistance | Medications |
|---|---|---|---|---|
|
|
Diagnosis
To diagnose gynecomastia, a thorough history and physical examination are obtained by a physician. Important aspects of the physical examination include evaluation of the male breast tissue with palpation to evaluate for breast cancer and pseudogynecomastia (male breast tissue enlargement solely due to excess fatty tissue), evaluation of penile size and development, evaluation of testicular development and an assessment for masses that raise suspicion for testicular cancer, and proper development of secondary sex characteristics such as the amount and distribution of pubic and underarm hair. Gynecomastia usually presents with bilateral involvement of the breast tissue but may occur unilaterally as well.
Diagnosis of men with breast enlargement can be evaluated using an algorithm. A review of the medications or substances an individual takes may reveal the cause of gynecomastia. Recommended laboratory investigations to find the underlying cause of gynecomastia include tests for aspartate transaminase and alanine transaminase to rule out liver disease, serum creatinine to determine if kidney damage is present, and thyroid-stimulating hormone levels to evaluate for hyperthyroidism. If these initial laboratory tests fail to uncover the cause of gynecomastia, then additional tests to evaluate for an underlying hormonal balance due to hypogonadism or a testicular tumor should be checked including total and free levels of testosterone, luteinizing hormone, follicle stimulating hormone, estradiol, serum beta human chorionic gonadotropin (β-hCG), and prolactin.
High levels of prolactin are uncommon in people with gynecomastia. If β-hCG levels are abnormally high, then ultrasound of the testicles should be performed to check for signs of a hormone-secreting testicular tumor. Markers of testicular, adrenal, or other tumors such as urinary 17-ketosteroid or serum dehydroepiandrosterone may also be checked if there is evidence of hormonal imbalance on physical examination. If this evaluation does not reveal the cause of gynecomastia, then it is considered to be idiopathic gynecomastia (of unclear cause).
Differential diagnosis
While there can be many potential causes of male patients that present with increased breast tissue, differential diagnoses are most concerning for gynecomastia, pseudogynecomastia, and breast cancer (which is rare in men). Other potential causes of male breast enlargement such as mastitis, lipoma, sebaceous cyst, dermoid cyst, hematoma, metastasis, ductal ectasia, fat necrosis, or a hamartoma are typically excluded before making the diagnosis.
Imaging
Mammography is the method of choice for radiologic examination of male breast tissue in the diagnosis of gynecomastia when breast cancer is suspected on physical examination. If a mass/lump is felt during a physical exam some features of the lump that would point to malignancy would be painless, non moveable (fixed), irregularly shaped, and skin changes. Mammography is rarely indicated for men since breast cancer is an unlikely diagnosis. If mammography is performed and does not reveal findings suggestive of breast cancer, further imaging is not typically necessary. If a tumor of the adrenal glands or the testes is thought to be responsible for the gynecomastia, ultrasound examination of these structures may be performed.
Histology

Early histological features expected to be seen on examination of gynecomastic tissue attained by fine-needle aspiration biopsy include the following: proliferation and lengthening of the ducts, an increase in connective tissue, an increase in inflammation, and swelling surrounding the ducts, and an increase in fibroblasts in the connective tissue. Chronic gynecomastia may show different histological features such as increased connective tissue fibrosis, an increase in the number of ducts, less inflammation than in the acute stage of gynecomastia, increased subareolar fat, and hyalinization of the stroma. When surgery is performed, the gland is routinely sent to the lab to confirm the presence of gynecomastia and to check for tumors under a microscope. The utility of pathologic examination of breast tissue removed from male adolescent gynecomastia patients has recently been questioned due to the rarity of breast cancer in this population.
Classification
The spectrum of gynecomastia severity has been categorized into a grading system:
- Grade I: Minor enlargement, no skin excess
- Grade II: Moderate enlargement, no skin excess
- Grade III: Moderate enlargement, skin excess
- Grade IV: Marked enlargement, skin excess
Treatment
If the gynecomastia doesn't resolve on its own in two years, then medical treatment is necessary. The options are medication or surgical intervention.
Medication
Gynecomastia can respond well to medical treatment although it is usually only effective when done within the first two years after the start of male breast enlargement. Selective estrogen receptor modulators (SERMs) such as tamoxifen, raloxifene, and clomifene may be beneficial in the treatment of gynecomastia but are not approved by the Food and Drug Administration for use in gynecomastia. Clomifene seems to be less effective than tamoxifen or raloxifene. Tamoxifen may be used to treat gynecomastia in adults and of the medical treatments used, tamoxifen is the most effective. Recent studies have shown that treatment with tamoxifen may represent a safe and effective mode of treatment in cases of cosmetically disturbing or painful gynecomastia. Aromatase inhibitors (AIs) such as anastrozole have been used off-label for cases of gynecomastia occurring during puberty but are less effective than SERMs.
A few cases of gynecomastia caused by the rare disorders aromatase excess syndrome and Peutz–Jeghers syndrome have responded to treatment with AIs such as anastrozole. Androgens/anabolic steroids may be effective for gynecomastia. Testosterone itself may not be suitable to treat gynecomastia as it can be aromatized into estradiol, but nonaromatizable androgens like topical androstanolone (dihydrotestosterone) can be useful.
Surgery

If chronic gynecomastia does not respond to medical treatment, surgical removal of glandular breast tissue is usually required. The American Board of Cosmetic Surgery reports surgery is the "most effective known treatment for gynecomastia." Surgical treatment should be considered if the gynecomastia persists for more than 12 months, causes distress (ie physical discomfort or psychological distress), and is in the fibrotic stage. In adolescent males, it is recommended that surgery is postponed until puberty is completed (penile and testicular development should reach Tanner scale Stage V).
Surgical approaches to the treatment of gynecomastia include subcutaneous mastectomy, liposuction-assisted mastectomy, laser-assisted liposuction, and laser-lipolysis without liposuction. Complications of mastectomy may include hematoma, surgical wound infection, breast asymmetry, changes in sensation in the breast, necrosis of the areola or nipple, seroma, noticeable or painful scars, and contour deformities. In 2019, 24,123 male patients underwent surgical treatment for gynecomastia in the United States, accounting for a 19% increase since 2000. Thirty-five percent of those patients were between the ages of 20 and 29, and 60% were younger than age 29 at the time of the operation. At an average surgeon's fee of $4,123, gynecomastia surgery was also the 11th most costly male cosmetic surgery of 2019.
Others
Radiation therapy and tamoxifen have been shown to help prevent gynecomastia and breast pain from developing in prostate cancer patients who will be receiving androgen deprivation therapy. The efficacy of these treatments is limited once gynecomastia has occurred and are therefore most effective when used prophylactically.
In the United States, many insurance companies deny coverage for surgery for gynecomastia treatment or male breast reduction on the basis that it is a cosmetic procedure.
Prognosis
Gynecomastia itself is a benign finding. It does not confer a poor prognosis, for some patients with underlying pathologies such as testicular cancer the prognosis may be worse. The glandular tissue typically grows under the influence of hormonal stimulation and is often tender or painful. Furthermore, gynecomastia frequently presents social and psychological difficulties such as low self-esteem, depression or shame.
Epidemiology
Gynecomastia is the most common benign disorder of the male breast tissue and affects 35 percent of men, being most prevalent between the ages of 50 and 69.
New cases of gynecomastia are common in three age populations: newborns, adolescents, and men older than 50 years. Newborn gynecomastia occurs in about 60–90 percent of male babies and most cases resolve on their own in about 2–3 weeks after delivery. During adolescence, on average 33 percent of males are estimated to exhibit signs of gynecomastia. Gynecomastia in older men is estimated to be present in 24–65 percent of men between the ages of 50 and 80. Estimates on asymptomatic gynecomastia is about up to 70% in men aged 50 to 69 years.
The prevalence of gynecomastia in men may have increased in recent years, but the epidemiology of the disorder is not fully understood. The use of anabolic steroids and exposure to chemicals that mimic estrogen in cosmetic products, organochlorine pesticides, and industrial chemicals have been suggested as possible factors driving this increase. According to the American Society of Plastic Surgeons, breast reduction surgeries to correct gynecomastia are fairly common but has been a recent decline. In 2020, there were over 18,000 procedures of this type performed in the United States which is down 11% compared to in 2019.
History
The term gynaecomastia was coined by Galen. He also recognised glandular enlargement of the male breast; however, this wasn't a condition of gynaecomastia according to him. A surgical procedure for treatment of gynaecomastia was described by Albucasis in his second book of Kitab al-Tasrif.
Society and culture
Gynecomastia can result in psychological distress for those with the condition. Support groups exist to help improve the self-esteem of affected people.
Moob, a portmanteau of man and boob, is a popular term to refer to male breasts. Use of anabolic steroids can result in gynecomastia, and male breasts are sometimes referred to using the pejorative bitch tits in bodybuilding communities.
In Murray v. Janssen Pharmaceuticals, Murray was a Risperidone user who was prescribed the medication at age nine and developed male breasts. A jury decided in Murray's favor in November 2015 and awarded him $1.75 million. The $1.75 million jury verdict represented damages for "disfigurement and mental anguish," though it was later reduced to $680,000. In the second portion of the bifurcated trial, the plaintiffs sought to prove that the companies knew and deliberately disregarded evidence that Risperidone could lead to gynecomastia in young males, and nonetheless promoted the medication off-label and released the medication into the open market for prescription and use by patients without disclosing the side effects. The jury found for the plaintiffs in the second portion of the trial and awarded $8 billion in punitive damages. The amount was later reduced to $6.8 million by Judge Kenneth Powell Jr.
In 2019, a 12-person Philadelphia jury awarded $8 billion in punitive damages to plaintiffs tied to the use of risperidone. Risperidone is an atypical antipsychotic that was originally approved to treat psychosis, but its use in children, including those with autism, ADHD, and schizophrenia diagnoses, has grown over the last two decades.
Etymology
The term comes from Greek γυνή gyné (stem gynaik-) 'female' and μαστός mastós 'breast'.
See also
References
- Explanatory notes
- The term comes from the Greek γυνή gyné (stem gynaik-) meaning "female" and μαστός mastós meaning "breast".
- Citations
- "Gynaecomastia | Definition of Gynaecomastia by Oxford Dictionary on Lexico.com". Lexico Dictionaries | English. OxfordDictionaries.com. Archived from the original on 8 March 2021. Retrieved 17 November 2020.
- "Definition of Gynecomastia". www.merriam-webster.com. Retrieved 17 November 2020.
- ^ Iuanow, Elaine; Kettler, Mark; Slanetz, Priscilla J. (March 2011). "Spectrum of Disease in the Male Breast". American Journal of Roentgenology. 196 (3): W247–W259. doi:10.2214/AJR.09.3994. PMID 21343472.
- ^ "Breast enlargement in males: MedlinePlus Medical Encyclopedia". medlineplus.gov. 2018. Retrieved 25 November 2020. Updated by Brent Wisse (10 November 2018)
- ^ Thiruchelvam, Paul; Walker, Jonathan Neil; Rose, Katy; Lewis, Jacqueline; Al-Mufti, Ragheed (22 September 2016). "Gynaecomastia". BMJ. 354: i4833. doi:10.1136/bmj.i4833. PMID 27659195. S2CID 220098795.
- ^ Niewoehner, CB; Schorer, AE (March 2008). "Gynaecomastia and breast cancer in men". British Medical Journal. 336 (7646): 709–713. doi:10.1136/bmj.39511.493391.BE. PMC 2276281. PMID 18369226.
- ^ Narula, Harmeet S.; Carlson, Harold E. (November 2014). "Gynaecomastia—pathophysiology, diagnosis and treatment". Nature Reviews Endocrinology. 10 (11): 684–698. doi:10.1038/nrendo.2014.139. PMID 25112235. S2CID 40159424.
- ^ Johnson RE, Murad MH (November 2009). "Gynecomastia: pathophysiology, evaluation, and management". Mayo Clinic Proceedings. 84 (11): 1010–1015. doi:10.4065/84.11.1010. PMC 2770912. PMID 19880691.
- ^ Chau, A; Jafarian, N; Rosa, M (February 2016). "Male Breast: Clinical and Imaging Evaluations of Benign and Malignant Entities with Histologic Correlation". The American Journal of Medicine (Review). 129 (8): 776–91. doi:10.1016/j.amjmed.2016.01.009. PMID 26844632.
- ^ Shulman, D. I.; Francis, G. L.; Palmert, M. R.; Eugster, E. A.; Lawson Wilkins Pediatric Endocrine Society Drug and Therapeutics, Committee. (1 April 2008). "Use of Aromatase Inhibitors in Children and Adolescents With Disorders of Growth and Adolescent Development". Pediatrics. 121 (4): e975–e983. doi:10.1542/peds.2007-2081. PMID 18381525. S2CID 39852740.
- "Gynecomastia: Surgery, treatment, causes, and symptoms". www.medicalnewstoday.com. 11 January 2018. Retrieved 16 April 2021.
- Dickson, Gretchen (1 April 2012). "Gynecomastia". American Family Physician. 85 (7): 716–722. ISSN 0002-838X. PMID 22534349.
- ^ Z. Hochberg (1 January 2007). Practical Algorithms in Pediatric Endocrinology. Karger Medical and Scientific Publishers. pp. 21–. ISBN 978-3-8055-8220-9.
- ^ Seth Thaller; Mimis Cohen (28 February 2013). Cosmetic Surgery After Massive Weight Loss. JP Medical Ltd. pp. 133–. ISBN 978-1-907816-28-4.
- de Ronde, W., de Jong, F.H. Aromatase inhibitors in men: effects and therapeutic options. Reprod Biol Endocrinol 9, 93 (2011). https://doi.org/10.1186/1477-7827-9-93
- ^ Wit, Jan M.; Hero, Matti; Nunez, Susan B. (March 2012). "Aromatase inhibitors in pediatrics". Nature Reviews Endocrinology. 8 (3): 135–147. doi:10.1038/nrendo.2011.161. PMID 22024975. S2CID 21710403.
- ^ Deepinder F, Braunstein GD (2012). "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety. 11 (5): 779–795. doi:10.1517/14740338.2012.712109. PMID 22862307. S2CID 22938364.
- ^ "Plastic Surgery Statistics Report" (PDF). American Society of Plastic Surgeons. 2019. Archived from the original (PDF) on 19 February 2021.
- Mark Dennis; William Talbot Bowen; Lucy Cho (31 August 2016). Mechanisms of Clinical Signs - EPub3. Elsevier Health Sciences. pp. 599–. ISBN 978-0-7295-8561-3.
- William T. O'Donohue; Lorraine T. Benuto; Lauren Woodward Tolle (8 July 2014). Handbook of Adolescent Health Psychology. Springer Science & Business Media. pp. 246–. ISBN 978-1-4614-6633-8.
- ^ Braunstein, Glenn D. (20 September 2007). "Gynecomastia". New England Journal of Medicine. 357 (12): 1229–1237. doi:10.1056/NEJMcp070677. ISSN 0028-4793. PMID 17881754.
- ^ Devalia HL, Layer GT (April 2009). "Current concepts in gynaecomastia". Surgeon. 7 (2): 114–19. doi:10.1016/s1479-666x(09)80026-7. PMID 19408804.
- Bell, Brenda K.; Rothman, Micol S. (1 January 2009), McDermott, Michael T. (ed.), "Chapter 46 - Gynecomastia", Endocrine Secrets (Fifth Edition), Philadelphia: Mosby, pp. 394–398, doi:10.1016/b978-0-323-05885-8.00046-5, ISBN 978-0-323-05885-8, retrieved 10 September 2022
- ^ Cordova A, Moschella F (2008). "Algorithm for clinical evaluation and surgical treatment of gynaecomastia". J Plast Reconstr Aesthet Surg. 61 (1): 41–9. doi:10.1016/j.bjps.2007.09.033. PMID 17983883.
- Rew, Lynn; Young, Cara; Harrison, Tracie; Caridi, Robert (August 2015). "A systematic review of literature on psychosocial aspects of gynecomastia in adolescents and young men". Journal of Adolescence. 43 (1): 206–212. doi:10.1016/j.adolescence.2015.06.007. ISSN 0140-1971. PMID 26151806.
- ^ Cuhaci N, Polat SB, Evranos B, Ersoy R, Cakir B (19 March 2014). "Gynecomastia: Clinical evaluation and management". Indian J Endocrinol Metab. 18 (2): 150–58. doi:10.4103/2230-8210.129104. PMC 3987263. PMID 24741509.
- ^ Dickson, G (1 April 2012). "Gynecomastia". American Family Physician. 85 (7): 716–22. PMID 22534349.
- Ordaz, D. Luis; Thompson, J. Kevin (September 2015). "Gynecomastia and psychological functioning: A review of the literature". Body Image. 15: 141–148. doi:10.1016/j.bodyim.2015.08.004.
- ^ Swerdloff, Ronald S.; Ng, Chiu Ming (2000), Feingold, Kenneth R.; Anawalt, Bradley; Boyce, Alison; Chrousos, George (eds.), "Gynecomastia: Etiology, Diagnosis, and Treatment", Endotext, South Dartmouth (MA): MDText.com, Inc., PMID 25905330, retrieved 9 September 2022
- Fleisher, Gary (2010). Textbook of pediatric emergency medicine (6th ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health. p. 731. ISBN 978-1-60547-159-4.
- Melmed, Shlomo (2011). Williams Textbook of Endocrinology: Expert Consult. Elsevier Health Sciences. pp. Chapter 19. ISBN 978-1-4377-3600-7.
- ^ Soliman, Ashraf T; De Sanctis, Vincenzo; Yassin, Mohamed (23 August 2017). "Management of Adolescent Gynecomastia: An Update". Acta Bio Medica: Atenei Parmensis. 88 (2): 204–213. doi:10.23750/abm.v88i2.6665. PMC 6166145. PMID 28845839.
- Nordt, Christina A; DiVasta, Amy D (August 2008). "Gynecomastia in adolescents". Current Opinion in Pediatrics. 20 (4): 375–382. doi:10.1097/MOP.0b013e328306a07c. ISSN 1040-8703. PMID 18622190. S2CID 205834072.
- Farida, Chentli; Faiza, Belhimer (2013). "Severe gynecomastia due to anti androgens intake: A case report and literature review". Indian Journal of Endocrinology and Metabolism. 17 (4): 730–2. doi:10.4103/2230-8210.113770. PMC 3743379. PMID 23961495.
- Gillatt, David (August 2006). "Antiandrogen treatments in locally advanced prostate cancer: are they all the same?". Journal of Cancer Research and Clinical Oncology. 132 (S1): 17–26. doi:10.1007/s00432-006-0133-5. PMID 16845534. S2CID 23888640.
- Goldspiel, Barry R.; Kohler, David R. (June 1990). "Flutamide: An Antiandrogen for Advanced Prostate Cancer". DICP. 24 (6): 616–623. doi:10.1177/106002809002400612. PMID 2193461. S2CID 26125621.
- Chung, Edmund YM; Ruospo, Marinella; Natale, Patrizia; Bolignano, Davide; Navaneethan, Sankar D; Palmer, Suetonia C; Strippoli, Giovanni FM (27 October 2020). "Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease". Cochrane Database of Systematic Reviews. 2020 (10): CD007004. doi:10.1002/14651858.CD007004.pub4. PMC 8094274. PMID 33107592.
- Aiman, U; Haseen, MA; Rahman, SZ (December 2009). "Gynecomastia: An ADR due to drug interaction". Indian Journal of Pharmacology. 41 (6): 286–287. doi:10.4103/0253-7613.59929. PMC 2846505. PMID 20407562.
- Nieschlag, Eberhard; Vorona, Elena (August 2015). "MECHANISMS IN ENDOCRINOLOGY: Medical consequences of doping with anabolic androgenic steroids: effects on reproductive functions". European Journal of Endocrinology. 173 (2): R47–R58. doi:10.1530/EJE-15-0080. PMID 25805894.
- ^ Barros, Alfredo Carlos Simões Dornellas de; Sampaio, Marcelo de Castro Moura (2012). "Gynecomastia: physiopathology, evaluation and treatment". Sao Paulo Medical Journal. 130 (3): 187–197. doi:10.1590/s1516-31802012000300009. PMC 10876201. PMID 22790552.
- Diaz A, Luque L, Badar Z, Kornic S, Danon M (2016). "Prepubertal gynecomastia and chronic lavender exposure: report of three cases". J. Pediatr. Endocrinol. Metab. 29 (1): 103–107. doi:10.1515/jpem-2015-0248. PMID 26353172. S2CID 19454282.
- Poon SW, Siu KK, Tsang AM (October 2020). "Isoniazid-induced gynaecomastia: report of a paediatric case and review of literature". BMC Endocr Disord (Review). 20 (1): 160. doi:10.1186/s12902-020-00639-9. PMC 7590456. PMID 33109161.
- Restrepo R, Cervantes LF, Swirsky AM, Diaz A (October 2021). "Breast development in pediatric patients from birth to puberty: physiology, pathology and imaging correlation". Pediatr Radiol (Review). 51 (11): 1959–1969. doi:10.1007/s00247-021-05099-4. PMID 34236480. S2CID 235767694.
- ^ Bembo, S. A; Carlson, H. E (1 June 2004). "Gynecomastia: its features, and when and how to treat it". Cleveland Clinic Journal of Medicine. 71 (6): 511–517. doi:10.3949/ccjm.71.6.511. ISSN 0891-1150. PMID 15242307. S2CID 21672450.
- Iglesias, P; Carrero, JJ; Díez, JJ (January 2012). "Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options". Journal of Nephrology. 25 (1): 31–42. doi:10.5301/JN.2011.8481. PMID 21748720.
- Grunseich, C; Fischbeck, KH (November 2015). "Spinal and Bulbar Muscular Atrophy". Neurologic Clinics. 33 (4): 847–54. doi:10.1016/j.ncl.2015.07.002. PMC 4628725. PMID 26515625.
- Fukami, M; Miyado, M; Nagasaki, K; Shozu, M; Ogata, T (March 2014). "Aromatase excess syndrome: a rare autosomal dominant disorder leading to pre- or peri-pubertal onset gynecomastia". Pediatric Endocrinology Reviews. 11 (3): 298–305. PMID 24716396.
- Gourgari, E; Saloustros, E; Stratakis, CA (August 2012). "Large-cell calcifying Sertoli cell tumors of the testes in pediatrics". Current Opinion in Pediatrics. 24 (4): 518–522. doi:10.1097/MOP.0b013e328355a279. PMC 4132931. PMID 22732638.
- Saylor, PJ; Smith, MR (May 2009). "Metabolic complications of androgen deprivation therapy for prostate cancer". The Journal of Urology. 181 (5): 1998–2006. doi:10.1016/j.juro.2009.01.047. PMC 2900631. PMID 19286225.
- ^ Johnson, Ruth E.; Murad, M. Hassan (November 2009). "Gynecomastia: Pathophysiology, Evaluation, and Management". Mayo Clinic Proceedings. 84 (11): 1010–1015. doi:10.1016/S0025-6196(11)60671-X. PMC 2770912. PMID 19880691.
- ^ Ismail, A. A.; Barth, J. H. (2001). "Endocrinology of gynaecomastia". Annals of Clinical Biochemistry. 38 (Pt 6): 596–607. doi:10.1258/0004563011900993. ISSN 0004-5632. PMID 11732643. S2CID 22948077.
- Gourgari, Evgenia; Saloustros, Emmanouil; Stratakis, Constantine A. (August 2012). "Large-cell calcifying Sertoli cell tumors of the testes in pediatrics". Current Opinion in Pediatrics. 24 (4): 518–522. doi:10.1097/MOP.0b013e328355a279. ISSN 1040-8703. PMC 4132931. PMID 22732638.
- Gies I, Unuane D, Velkeniers B, De Schepper J (August 2014). "Management of Klinefelter syndrome during transition". European Journal of Endocrinology. 171 (2): R67–77. doi:10.1530/EJE-14-0213. PMID 24801585.
- ^ Hughes, Ieuan A.; Deeb, Asma (1 December 2006). "Androgen resistance". Best Practice & Research Clinical Endocrinology & Metabolism. Hormone Resistance Syndromes. 20 (4): 577–598. doi:10.1016/j.beem.2006.11.003. ISSN 1521-690X. PMID 17161333.
- Ali, Omar (2020), "Gynecomastia", Nelson Textbook of Pediatrics, Elsevier, pp. 3000–3001.e1, ISBN 9780323529501
- Mayo Clinic Staff (2010). "Tests and diagnosis". Mayo Clinic. Retrieved 3 February 2013.
- ^ Koshy, John C.; Goldberg, Jonathan S.; Wolfswinkel, Eric M.; Ge, Yimin; Heller, Lior (January 2011). "Breast Cancer Incidence in Adolescent Males Undergoing Subcutaneous Mastectomy for Gynecomastia: Is Pathologic Examination Justified? A Retrospective and Literature Review". Plastic and Reconstructive Surgery. 127 (1): 1–7. doi:10.1097/PRS.0b013e3181f9581c. PMID 20871489. S2CID 43048316.
- ^ Wollina, Uwe; Goldman, Alberto (June 2011). "Minimally invasive esthetic procedures of the male breast: Male breast". Journal of Cosmetic Dermatology. 10 (2): 150–155. doi:10.1111/j.1473-2165.2011.00548.x. PMID 21649820. S2CID 39542596.
- "Enlarged breasts in men (gynecomastia) - Diagnosis and treatment - Mayo Clinic". www.mayoclinic.org. Retrieved 29 October 2022.
- ^ Agrawal, Sweety; Ganie, Mohd Ashraf; Nisar, Sobia (2017). "Gynaecomastia". Basics of Human Andrology. pp. 451–458. doi:10.1007/978-981-10-3695-8_26. ISBN 978-981-10-3694-1.
- Kunath, Frank; Keck, Bastian; Antes, Gerd; Wullich, Bernd; Meerpohl, Joerg J (December 2012). "Tamoxifen for the management of breast events induced by non-steroidal antiandrogens in patients with prostate cancer: a systematic review". BMC Medicine. 10 (1): 96. doi:10.1186/1741-7015-10-96. PMC 3464149. PMID 22925442.
- "Gynaecomastia. Information about Gynaecomastia". 21 July 2021.
- Parker, Lawrence N.; Gray, David R.; Lai, Michael K.; Levin, Ellis R. (August 1986). "Treatment of gynecomastia with tamoxifen: A double-blind crossover study". Metabolism. 35 (8): 705–708. doi:10.1016/0026-0495(86)90237-4. PMID 3526085.
- "Male Breast Reduction Guide | Gynecomastia Surgery Guide".
- ^ "UpToDate". www.uptodate.com. Retrieved 12 September 2022.
- Viani, Gustavo Arruda; Bernardes da Silva, Lucas Godói; Stefano, Eduardo Jose (July 2012). "Prevention of Gynecomastia and Breast Pain Caused by Androgen Deprivation Therapy in Prostate Cancer: Tamoxifen or Radiotherapy?". International Journal of Radiation Oncology, Biology, Physics. 83 (4): e519–e524. doi:10.1016/j.ijrobp.2012.01.036. PMID 22704706.
- "Coverage Determination Guideline Gynecomastia Treatment" (PDF). United HealthCare Services, Inc. 2012. Archived from the original (PDF) on 19 April 2014. Retrieved 6 May 2019.
- "Clinical Policy Bulletin: Breast Reduction Surgery and Gynecomastia Surgery". Aetna Inc. 2012. Retrieved 12 February 2013.are
- "Cigna Medical Coverage Policy" (PDF). Surgical Treatment of Gynecomastia. Cigna. 2012. Archived from the original (PDF) on 5 September 2012. Retrieved 12 February 2013.
- ^ Wassersug, Richard J.; Oliffe, John L. (April 2009). "The Social Context for Psychological Distress from Iatrogenic Gynecomastia with Suggestions for Its Management". The Journal of Sexual Medicine. 6 (4): 989–1000. doi:10.1111/j.1743-6109.2008.01053.x. PMID 19175864.
- Georgiade, Nicholas G. (1976). Reconstructive Breast Surgery. Mosby. ISBN 978-0-8016-1802-4.
- Chavoushi, Seyed Hadi; Ghabili, Kamyar; Kazemi, Abdolhassan; Aslanabadi, Arash; Babapour, Sarah; Ahmedli, Rafail; Golzari, Samad E. J. (2012). "Surgery for Gynecomastia in the Islamic Golden Age: Al-Tasrif of Al-Zahrawi (936-1013 AD)". ISRN Surgery. 2012: 934965. doi:10.5402/2012/934965. ISSN 2090-5793. PMC 3459224. PMID 23050167.
- Smith, Merrill D (2014). Cultural Encyclopedia of the Breast. Rowman & Littlefield. p. 124. ISBN 978-0-7591-2332-8.
- ^ Malfitano, Nicholas (8 October 2019). "In first test, Philadelphia jury punishes J&J with an $8 billion verdict; Thousands more cases remain". Pennsylvania Record. Retrieved 18 January 2019.
- Malfitano, Nicholas (17 January 2020). "Judge in landmark $8 billion Risperdal case cuts punitive damages award down to $6.8 million". Pennsylvania Record. Retrieved 18 January 2019.
- Miller, Caroline. "What Parents Should Know About Risperdal". Child Mind Institute. Retrieved 18 January 2020.
External links
 Media related to Gynecomastia at Wikimedia Commons
Media related to Gynecomastia at Wikimedia Commons
| Classification | D |
|---|---|
| External resources |
| Breast disease | |
|---|---|
| Inflammation | |
| Physiological changes and conditions | |
| Nipple | |
| Masses | |
| Other | |