Type of intracranial bleeding that occurs within the brain tissue itself
Medical condition
| Intracerebral hemorrhage | |
|---|---|
| Other names | Cerebral haemorrhage, cerebral hemorrhage, intra-axial hemorrhage, cerebral hematoma, cerebral bleed, brain bleed, hemorrhagic stroke |
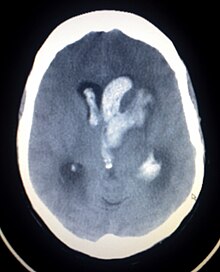 | |
| CT scan of a spontaneous intracerebral bleed, leaking into the lateral ventricles | |
| Specialty | Neurosurgery |
| Symptoms | Headache, one-sided numbness, weakness, tingling, or paralysis, speech problems, vision or hearing problems, dizziness or lightheadedness or vertigo, nausea/vomiting, seizures, decreased level or total loss of consciousness, neck stiffness, memory loss, attention and coordination problems, balance problems, fever, shortness of breath (when bleed is in the brain stem) |
| Complications | Coma, persistent vegetative state, cardiac arrest (when bleeding is severe or in the brain stem), death |
| Causes | Brain trauma, aneurysms, arteriovenous malformations, brain tumors, hemorrhagic conversion of ischemic stroke |
| Risk factors | High blood pressure, diabetes, high cholesterol, amyloidosis, alcoholism, low cholesterol, blood thinners, cocaine use |
| Diagnostic method | CT scan |
| Differential diagnosis | Ischemic stroke |
| Treatment | Blood pressure control, surgery, ventricular drain |
| Prognosis | 20% good outcome |
| Frequency | 2.5 per 10,000 people a year |
| Deaths | 44% die within one month |
Intracerebral hemorrhage (ICH), also known as hemorrhagic stroke, is a sudden bleeding into the tissues of the brain (i.e. the parenchyma), into its ventricles, or into both. An ICH is a type of bleeding within the skull and one kind of stroke (ischemic stroke being the other). Symptoms can vary dramatically depending on the severity (how much blood), acuity (over what timeframe), and location (anatomically) but can include headache, one-sided weakness, numbness, tingling, or paralysis, speech problems, vision or hearing problems, memory loss, attention problems, coordination problems, balance problems, dizziness or lightheadedness or vertigo, nausea/vomiting, seizures, decreased level of consciousness or total loss of consciousness, neck stiffness, and fever.
Hemorrhagic stroke may occur on the background of alterations to the blood vessels in the brain, such as cerebral arteriolosclerosis, cerebral amyloid angiopathy, cerebral arteriovenous malformation, brain trauma, brain tumors and an intracranial aneurysm, which can cause intraparenchymal or subarachnoid hemorrhage.
The biggest risk factors for spontaneous bleeding are high blood pressure and amyloidosis. Other risk factors include alcoholism, low cholesterol, blood thinners, and cocaine use. Diagnosis is typically by CT scan.
Treatment should typically be carried out in an intensive care unit due to strict blood pressure goals and frequent use of both pressors and antihypertensive agents. Anticoagulation should be reversed if possible and blood sugar kept in the normal range. A procedure to place an external ventricular drain may be used to treat hydrocephalus or increased intracranial pressure, however, the use of corticosteroids is frequently avoided. Sometimes surgery to directly remove the blood can be therapeutic.
Cerebral bleeding affects about 2.5 per 10,000 people each year. It occurs more often in males and older people. About 44% of those affected die within a month. A good outcome occurs in about 20% of those affected. Intracerebral hemorrhage, a type of hemorrhagic stroke, was first distinguished from ischemic strokes due to insufficient blood flow, so called "leaks and plugs", in 1823.
Epidemiology
The incidence of intracerebral hemorrhage is estimated at 24.6 cases per 100,000 person years with the incidence rate being similar in men and women. The incidence is much higher in the elderly, especially those who are 85 or older, who are 9.6 times more likely to have an intracerebral hemorrhage as compared to those of middle age. It accounts for 20% of all cases of cerebrovascular disease in the United States, behind cerebral thrombosis (40%) and cerebral embolism (30%).
Types
See also: Subarachnoid hemorrhageIntraparenchymal hemorrhage
Intraparenchymal hemorrhage (IPH) is one form of intracerebral bleeding in which there is bleeding within brain parenchyma. Intraparenchymal hemorrhage accounts for approximately 8-13% of all strokes and results from a wide spectrum of disorders. It is more likely to result in death or major disability than ischemic stroke or subarachnoid hemorrhage, and therefore constitutes an immediate medical emergency. Intracerebral hemorrhages and accompanying edema may disrupt or compress adjacent brain tissue, leading to neurological dysfunction. Substantial displacement of brain parenchyma may cause elevation of intracranial pressure (ICP) and potentially fatal herniation syndromes.
Intraventricular hemorrhage
Intraventricular hemorrhage (IVH), also known asintraventricular bleeding, is a bleeding into the brain's ventricular system, where the cerebrospinal fluid is produced and circulates through towards the subarachnoid space. It can result from physical trauma or from hemorrhagic stroke.
30% of intraventricular hemorrhage (IVH) are primary, confined to the ventricular system and typically caused by intraventricular trauma, aneurysm, vascular malformations, or tumors, particularly of the choroid plexus. However 70% of IVH are secondary in nature, resulting from an expansion of an existing intraparenchymal or subarachnoid hemorrhage. Intraventricular hemorrhage has been found to occur in 35% of moderate to severe traumatic brain injuries. Thus the hemorrhage usually does not occur without extensive associated damage, and so the outcome is rarely good.
Signs and symptoms
People with intracerebral bleeding have symptoms that correspond to the functions controlled by the area of the brain that is damaged by the bleed. These localizing signs and symptoms can include hemiplegia (or weakness localized to one side of the body) and paresthesia (loss of sensation) including hemisensory loss (if localized to one side of the body). These symptoms are usually rapid in onset, sometimes occurring in minutes, but not as rapid as the symptom onset in ischemic stroke. While the duration of onset not be as rapid, it is important that patients go to the emergency department as soon as they notice any symptoms as early detection and management of stroke may lead to better outcomes post-stroke than delayed identification.
A mnemonic to remember the warning signs of stroke is FAST (facial droop, arm weakness, speech difficulty, and time to call emergency services), as advocated by the Department of Health (United Kingdom) and the Stroke Association, the American Stroke Association, the National Stroke Association (US), the Los Angeles Prehospital Stroke Screen (LAPSS) and the Cincinnati Prehospital Stroke Scale (CPSS). Use of these scales is recommended by professional guidelines. FAST is less reliable in the recognition of posterior circulation stroke.
Other symptoms include those that indicate a rise in intracranial pressure caused by a large mass (due to hematoma expansion) putting pressure on the brain. These symptoms include headaches, nausea, vomiting, a depressed level of consciousness, stupor and death. Continued elevation in the intracranial pressure and the accompanying mass effect may eventually cause brain herniation (when different parts of the brain are displaced or shifted to new areas in relation to the skull and surrounding dura mater supporting structures). Brain herniation is associated with hyperventilation, extensor rigidity, pupillary asymmetry, pyramidal signs, coma and death.
Hemorrhage into the basal ganglia or thalamus causes contralateral hemiplegia due to damage to the internal capsule. Other possible symptoms include gaze palsies or hemisensory loss. Intracerebral hemorrhage into the cerebellum may cause ataxia, vertigo, incoordination of limbs and vomiting. Some cases of cerebellar hemorrhage lead to blockage of the fourth ventricle with subsequent impairment of drainage of cerebrospinal fluid from the brain. The ensuing hydrocephalus, or fluid buildup in the ventricles of the brain leads to a decreased level of consciousness, total loss of consciousness, coma, and persistent vegetative state. Brainstem hemorrhage most commonly occurs in the pons and is associated with shortness of breath, cranial nerve palsies, pinpoint (but reactive) pupils, gaze palsies, facial weakness, coma, and persistent vegetative state (if there is damage to the reticular activating system).
Causes
Intracerebral bleeds are the second most common cause of stroke, accounting for 10% of hospital admissions for stroke. High blood pressure raises the risks of spontaneous intracerebral hemorrhage by two to six times. More common in adults than in children, intraparenchymal bleeds are usually due to penetrating head trauma, but can also be due to depressed skull fractures. Acceleration-deceleration trauma, rupture of an aneurysm or arteriovenous malformation (AVM), and bleeding within a tumor are additional causes. Amyloid angiopathy is not an uncommon cause of intracerebral hemorrhage in patients over the age of 55. A very small proportion is due to cerebral venous sinus thrombosis.
Risk factors for ICH include:
- Hypertension (high blood pressure)
- Diabetes mellitus
- Menopause
- Excessive alcohol consumption
- Severe migraine
Hypertension is the strongest risk factor associated with intracerebral hemorrhage and long term control of elevated blood pressure has been shown to reduce the incidence of hemorrhage. Cerebral amyloid angiopathy, a disease characterized by deposition of amyloid beta peptides in the walls of the small blood vessels of the brain, leading to weakened blood vessel walls and an increased risk of bleeding; is also an important risk factor for the development of intracerebral hemorrhage. Other risk factors include advancing age (usually with a concomitant increase of cerebral amyloid angiopathy risk in the elderly), use of anticoagulants or antiplatelet medications, the presence of cerebral microbleeds, chronic kidney disease, and low low density lipoprotein (LDL) levels (usually below 70). The direct oral anticoagulants (DOACs) such as the factor Xa inhibitors or direct thrombin inhibitors are thought to have a lower risk of intracerebral hemorrhage as compared to the vitamin K antagonists such as warfarin.
Cigarette smoking may be a risk factor but the association is weak.
Traumautic intracerebral hematomas are divided into acute and delayed. Acute intracerebral hematomas occur at the time of the injury while delayed intracerebral hematomas have been reported from as early as 6 hours post injury to as long as several weeks.
Diagnosis

Both computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have been proved to be effective in diagnosing intracranial vascular malformations after ICH. So frequently, a CT angiogram will be performed in order to exclude a secondary cause of hemorrhage or to detect a "spot sign".
Intraparenchymal hemorrhage can be recognized on CT scans because blood appears brighter than other tissue and is separated from the inner table of the skull by brain tissue. The tissue surrounding a bleed is often less dense than the rest of the brain because of edema, and therefore shows up darker on the CT scan. The oedema surrounding the haemorrhage would rapidly increase in size in the first 48 hours, and reached its maximum extent at day 14. The bigger the size of the haematoma, the larger its surrounding oedema. Brain oedema formation is due to the breakdown of red blood cells, where haemoglobin and other contents of red blood cells are released. The release of these red blood cells contents causes toxic effect on the brain and causes brain oedema. Besides, the breaking down of blood-brain barrier also contributes to the odema formation.
Apart from CT scans, haematoma progression of intracerebral haemorrhage can be monitored using transcranial ultrasound. Ultrasound probe can be placed at the temporal lobe to estimate the volume of haematoma within the brain, thus identifying those with active bleeding for further intervention to stop the bleeding. Using ultrasound can also reduces radiation risk to the subject from CT scans.
Location
When due to high blood pressure, intracerebral hemorrhages typically occur in the putamen (50%) or thalamus (15%), cerebrum (10–20%), cerebellum (10–13%), pons (7–15%), or elsewhere in the brainstem (1–6%).
Treatment
Treatment depends substantially on the type of ICH. Rapid CT scan and other diagnostic measures are used to determine proper treatment, which may include both medication and surgery.
- Tracheal intubation is indicated in people with decreased level of consciousness or other risk of airway obstruction.
- IV fluids are given to maintain fluid balance, using isotonic rather than hypotonic fluids.
Medications
Rapid lowering of the blood pressure using antihypertensive therapy for those with hypertensive emergency can have higher functional recovery at 90 days post intracerebral haemorrhage, when compared to those who undergone other treatments such as mannitol administration, reversal of anticoagulation (those previously on anticoagulant treatment for other conditions), surgery to evacuate the haematoma, and standard rehabilitation care in hospital, while showing similar rate of death at 12%. Early lowering of the blood pressure can reduce the volume of the haematoma, but may not have any effect against the oedema surrounding the haematoma. Reducing the blood pressure rapidly does not cause brain ischemia in those who have intracerebral haemorrhage. The American Heart Association and American Stroke Association guidelines in 2015 recommended decreasing the blood pressure to a SBP of 140 mmHg. However, later reviews found unclear difference between intensive and less intensive blood pressure control.
Giving Factor VIIa within 4 hours limits the bleeding and formation of a hematoma. However, it also increases the risk of thromboembolism. It thus overall does not result in better outcomes in those without hemophilia.
Frozen plasma, vitamin K, protamine, or platelet transfusions may be given in case of a coagulopathy. Platelets however appear to worsen outcomes in those with spontaneous intracerebral bleeding on antiplatelet medication.
The specific reversal agents idarucizumab and andexanet alfa may be used to stop continued intracerebral hemorrhage in people taking directly oral acting anticoagulants (such as factor Xa inhibitors or direct thrombin inhibitors). However, if these specialized medications are not available, prothrombin complex concentrate may also be used.
Only 7% of those with ICH are presented with clinical features of seizures while up to 25% of those have subclinical seizures. Seizures are not associated with an increased risk of death or disability. Meanwhile, anticonvulsant administration can increase the risk of death. Therefore, anticonvulsants are only reserved for those that have shown obvious clinical features of seizures or seizure activity on electroencephalography (EEG).
H2 antagonists or proton pump inhibitors are commonly given to try to prevent stress ulcers, a condition linked with ICH.
Corticosteroids were thought to reduce swelling. However, in large controlled studies, corticosteroids have been found to increase mortality rates and are no longer recommended.
Surgery
Surgery is required if the hematoma is greater than 3 cm (1 in), if there is a structural vascular lesion or lobar hemorrhage in a young patient.
A catheter may be passed into the brain vasculature to close off or dilate blood vessels, avoiding invasive surgical procedures.
Aspiration by stereotactic surgery or endoscopic drainage may be used in basal ganglia hemorrhages, although successful reports are limited.
A craniectomy holds promise of reduced mortality, but the effects of long‐term neurological outcome remain controversial.
Prognosis
About 8 to 33% of those with intracranial haemorrhage have neurological deterioration within the first 24 hours of hospital admission, where a large proportion of them happens within 6 to 12 hours. Rate of haematoma expansion, perihaematoma odema volume and the presence of fever can affect the chances of getting neurological complications.
The risk of death from an intraparenchymal bleed in traumatic brain injury is especially high when the injury occurs in the brain stem. Intraparenchymal bleeds within the medulla oblongata are almost always fatal, because they cause damage to cranial nerve X, the vagus nerve, which plays an important role in blood circulation and breathing. This kind of hemorrhage can also occur in the cortex or subcortical areas, usually in the frontal or temporal lobes when due to head injury, and sometimes in the cerebellum. Larger volumes of hematoma at hospital admission as well as greater expansion of the hematoma on subsequent evaluation (usually occurring within 6 hours of symptom onset) are associated with a worse prognosis. Perihematomal edema, or secondary edema surrounding the hematoma, is associated with secondary brain injury, worsening neurological function and is associated with poor outcomes. Intraventricular hemorrhage, or bleeding into the ventricles of the brain, which may occur in 30–50% of patients, is also associated with long-term disability and a poor prognosis. Brain herniation is associated with poor prognoses.
For spontaneous intracerebral hemorrhage seen on CT scan, the death rate (mortality) is 34–50% by 30 days after the injury, and half of the deaths occur in the first 2 days. Even though the majority of deaths occur in the first few days after ICH, survivors have a long-term excess mortality rate of 27% compared to the general population. Of those who survive an intracerebral hemorrhage, 12–39% are independent with regard to self-care; others are disabled to varying degrees and require supportive care.
References
- ^ Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. (Council on Clinical Cardiology; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing) (July 2015). "Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association". Stroke. 46 (7): 2032–2060. doi:10.1161/str.0000000000000069. PMID 26022637.
- ^ Caceres JA, Goldstein JN (August 2012). "Intracranial hemorrhage". Emergency Medicine Clinics of North America. 30 (3): 771–794. doi:10.1016/j.emc.2012.06.003. PMC 3443867. PMID 22974648.
- ^ "Brain Bleed/Hemorrhage (Intracranial Hemorrhage): Causes, Symptoms, Treatment".
- ^ Naidich TP, Castillo M, Cha S, Smirniotopoulos JG (2012). Imaging of the Brain, Expert Radiology Series,1: Imaging of the Brain. Elsevier Health Sciences. p. 387. ISBN 978-1416050094. Archived from the original on 2016-10-02.
- Ko SB, Yoon BW (December 2017). "Blood Pressure Management for Acute Ischemic and Hemorrhagic Stroke: The Evidence". Seminars in Respiratory and Critical Care Medicine. 38 (6): 718–725. doi:10.1055/s-0037-1608777. PMID 29262429.
- Hennerici M (2003). Imaging in Stroke. Remedica. p. 1. ISBN 9781901346251. Archived from the original on 2016-10-02.
- ^ Sheth KN (October 2022). "Spontaneous Intracerebral Hemorrhage". The New England Journal of Medicine. 387 (17): 1589–1596. doi:10.1056/NEJMra2201449. PMID 36300975. S2CID 253159180.
- ^ van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ (February 2010). "Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis". The Lancet. Neurology. 9 (2): 167–176. doi:10.1016/S1474-4422(09)70340-0. PMID 20056489. S2CID 25364307.
- Page 117 in: Schutta HS, Lechtenberg R (1998). Neurology practice guidelines. New York: M. Dekker. ISBN 978-0-8247-0104-8.
- ^ Kalita J, Misra UK, Vajpeyee A, Phadke RV, Handique A, Salwani V (April 2009). "Brain herniations in patients with intracerebral hemorrhage". Acta Neurologica Scandinavica. 119 (4): 254–260. doi:10.1111/j.1600-0404.2008.01095.x. PMID 19053952. S2CID 21870062.
- ^ Feldmann E, Broderick JP, Kernan WN, Viscoli CM, Brass LM, Brott T, et al. (September 2005). "Major risk factors for intracerebral hemorrhage in the young are modifiable". Stroke. 36 (9): 1881–1885. doi:10.1161/01.str.0000177480.62341.6b. PMID 16081867.
- ^ Josephson CB, White PM, Krishan A, Al-Shahi Salman R (September 2014). "Computed tomography angiography or magnetic resonance angiography for detection of intracranial vascular malformations in patients with intracerebral haemorrhage". The Cochrane Database of Systematic Reviews. 2014 (9): CD009372. doi:10.1002/14651858.CD009372.pub2. PMC 6544803. PMID 25177839.
- ^ Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF, Hoff JT (December 2001). "Mechanisms of edema formation after intracerebral hemorrhage: effects of extravasated red blood cells on blood flow and blood-brain barrier integrity". Stroke. 32 (12): 2932–2938. doi:10.1161/hs1201.099820. PMID 11739998. S2CID 7089563.
- ^ Ovesen C, Christensen AF, Krieger DW, Rosenbaum S, Havsteen I, Christensen H (April 2014). "Time course of early postadmission hematoma expansion in spontaneous intracerebral hemorrhage". Stroke. 45 (4): 994–999. doi:10.1161/STROKEAHA.113.003608. PMID 24627116. S2CID 7716659.
- ^ Vinas FC, Pilitsis J (2006). "Penetrating Head Trauma". Emedicine.com. Archived from the original on 2005-09-13.
- Colton K, Richards CT, Pruitt PB, Mendelson SJ, Holl JL, Naidech AM, et al. (February 2020). "Early Stroke Recognition and Time-based Emergency Care Performance Metrics for Intracerebral Hemorrhage". Journal of Stroke and Cerebrovascular Diseases. 29 (2): 104552. doi:10.1016/j.jstrokecerebrovasdis.2019.104552. PMC 6954314. PMID 31839545.
- Harbison J, Massey A, Barnett L, Hodge D, Ford GA (June 1999). "Rapid ambulance protocol for acute stroke". Lancet. 353 (9168): 1935. doi:10.1016/S0140-6736(99)00966-6. PMID 10371574. S2CID 36692451.
- Kidwell CS, Saver JL, Schubert GB, Eckstein M, Starkman S (January 1998). "Design and retrospective analysis of the Los Angeles Prehospital Stroke Screen (LAPSS)". Prehospital Emergency Care. 2 (4): 267–273. doi:10.1080/10903129808958878. PMID 9799012.
- Kothari RU, Pancioli A, Liu T, Brott T, Broderick J (April 1999). "Cincinnati Prehospital Stroke Scale: reproducibility and validity". Annals of Emergency Medicine. 33 (4): 373–378. doi:10.1016/S0196-0644(99)70299-4. PMID 10092713.
- Meredith TL, Freed N, Soulsby C, Bay S (2009). "Clinical audit to assess implications of implementing National Institute for Health and Clinical Excellence (NICE) guideline 32: Department of Nutrition and Dietetics at Barts and the London National Health Service (NHS) Trust 2007". Proceedings of the Nutrition Society. 68 (OCE1). doi:10.1017/s0029665109002080. ISSN 0029-6651.
- Merwick Á, Werring D (May 2014). "Posterior circulation ischaemic stroke". BMJ. 348 (may19 33): g3175. doi:10.1136/bmj.g3175. PMID 24842277.
- ^ Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A (January 2007). "Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus". BMC Neurology. 7: 1. doi:10.1186/1471-2377-7-1. PMC 1780056. PMID 17204141.
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. (January 2013). "Heart disease and stroke statistics--2013 update: a report from the American Heart Association". Circulation. 127 (1): e6–e245. doi:10.1161/CIR.0b013e31828124ad. PMC 5408511. PMID 23239837.
- ^ McCaffrey P (2001). "CMSD 336 Neuropathologies of Language and Cognition". The Neuroscience on the Web Series. Chico: California State University. Archived from the original on 2005-11-25. Retrieved 19 June 2007.
- "Overview of Adult Traumatic Brain Injuries" (PDF). Orlando Regional Healthcare, Education and Development. 2004. Archived from the original (PDF) on 2008-02-27. Retrieved 2008-01-16.
- Shepherd S (2004). "Head Trauma". Emedicine.com. Archived from the original on 2005-10-26. Retrieved 19 June 2007.
- Ma C, Gurol ME, Huang Z, Lichtenstein AH, Wang X, Wang Y, et al. (July 2019). "Low-density lipoprotein cholesterol and risk of intracerebral hemorrhage: A prospective study". Neurology. 93 (5): e445–e457. doi:10.1212/WNL.0000000000007853. PMC 6693427. PMID 31266905.
- An SJ, Kim TJ, Yoon BW (January 2017). "Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update". Journal of Stroke. 19 (1): 3–10. doi:10.5853/jos.2016.00864. PMC 5307940. PMID 28178408.
- Carhuapoma JR, Mayer SA, Hanley DF (2009). Intracerebral Hemorrhage. Cambridge University Press. p. 6. ISBN 978-0-521-87331-4.
- ^ Yeung R, Ahmad T, Aviv RI, de Tilly LN, Fox AJ, Symons SP (March 2009). "Comparison of CTA to DSA in determining the etiology of spontaneous ICH". The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques. 36 (2): 176–180. doi:10.1017/s0317167100006533. PMID 19378710.
- Venkatasubramanian C, Mlynash M, Finley-Caulfield A, Eyngorn I, Kalimuthu R, Snider RW, Wijman CA (January 2011). "Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging". Stroke. 42 (1): 73–80. doi:10.1161/STROKEAHA.110.590646. PMC 3074599. PMID 21164136.
- Greenberg MS (2016). Handbook of Neurosurgery. Thieme. ISBN 9781626232419.
- Prayson RA (2012). Neuropathology. Elsevier Health Sciences. p. 49. ISBN 978-1437709490. Archived from the original on 2017-03-12.
- ^ Liebeskind DS (7 August 2006). "Intracranial Haemorrhage: Treatment & Medication". eMedicine Specialties > Neurology > Neurological Emergencies. Archived from the original on 2009-03-12.
- Anderson CS, Qureshi AI (January 2015). "Implications of INTERACT2 and other clinical trials: blood pressure management in acute intracerebral hemorrhage". Stroke. 46 (1): 291–295. doi:10.1161/STROKEAHA.114.006321. PMID 25395408. S2CID 45730236.
- Anderson CS, Huang Y, Arima H, Heeley E, Skulina C, Parsons MW, et al. (February 2010). "Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT)". Stroke. 41 (2): 307–312. doi:10.1161/STROKEAHA.109.561795. PMID 20044534. S2CID 5871420.
- Butcher KS, Jeerakathil T, Hill M, Demchuk AM, Dowlatshahi D, Coutts SB, et al. (March 2013). "The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial". Stroke. 44 (3): 620–626. doi:10.1161/STROKEAHA.111.000188. PMID 23391776. S2CID 54488358.
- Ma J, Li H, Liu Y, You C, Huang S, Ma L (2015). "Effects of Intensive Blood Pressure Lowering on Intracerebral Hemorrhage Outcomes: A Meta-Analysis of Randomized Controlled Trials". Turkish Neurosurgery. 25 (4): 544–551. doi:10.5137/1019-5149.JTN.9270-13.0 (inactive 1 November 2024). PMID 26242330.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - Boulouis G, Morotti A, Goldstein JN, Charidimou A (April 2017). "Intensive blood pressure lowering in patients with acute intracerebral haemorrhage: clinical outcomes and haemorrhage expansion. Systematic review and meta-analysis of randomised trials". Journal of Neurology, Neurosurgery, and Psychiatry. 88 (4): 339–345. doi:10.1136/jnnp-2016-315346. PMID 28214798. S2CID 25397701.
- Yuan ZH, Jiang JK, Huang WD, Pan J, Zhu JY, Wang JZ (June 2010). "A meta-analysis of the efficacy and safety of recombinant activated factor VII for patients with acute intracerebral hemorrhage without hemophilia". Journal of Clinical Neuroscience. 17 (6): 685–693. doi:10.1016/j.jocn.2009.11.020. PMID 20399668. S2CID 30590573.
- Eilertsen H, Menon CS, Law ZK, Chen C, Bath PM, Steiner T, Desborough M Jr, Sandset EC, Sprigg N, Al-Shahi Salman R (2023-10-23). "Haemostatic therapies for stroke due to acute, spontaneous intracerebral haemorrhage". The Cochrane Database of Systematic Reviews. 2023 (10): CD005951. doi:10.1002/14651858.CD005951.pub5. ISSN 1469-493X. PMC 10591281. PMID 37870112.
- Fogarty Mack P (December 2014). "Intracranial haemorrhage: therapeutic interventions and anaesthetic management". British Journal of Anaesthesia. 113 (Suppl 2): ii17–ii25. doi:10.1093/bja/aeu397. PMID 25498578.
- Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. (9 October 2016). "Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial". Lancet. 364 (9442): 1321–1328. doi:10.1016/S0140-6736(04)17188-2. PMID 15474134. S2CID 30210176.
- Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, et al. (2005). "Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months". Lancet. 365 (9475): 1957–1959. doi:10.1016/s0140-6736(05)66552-x. PMID 15936423. S2CID 27713031.
- "Cerebral Hemorrhages". Cedars-Sinai Health System. Archived from the original on 2009-03-12. Retrieved 25 February 2009.
- Sahuquillo J, Dennis JA (December 2019). "Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury". The Cochrane Database of Systematic Reviews. 2019 (12): CD003983. doi:10.1002/14651858.CD003983.pub3. PMC 6953357. PMID 31887790.
- Lord AS, Gilmore E, Choi HA, Mayer SA (March 2015). "Time course and predictors of neurological deterioration after intracerebral hemorrhage". Stroke. 46 (3): 647–652. doi:10.1161/STROKEAHA.114.007704. PMC 4739782. PMID 25657190.
- Sanders MJ, McKenna K (2001). "Chapter 22: Head and Facial Trauma". Mosby's Paramedic Textbook (2nd revised ed.). Mosby.
- Graham DI, Gennareli TA (2000). "Chapter 5". In Cooper P, Golfinos G (eds.). Pathology of Brain Damage After Head Injury (4th ed.). New York: Morgan Hill.
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (July 1993). "Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality". Stroke. 24 (7): 987–993. doi:10.1161/01.STR.24.7.987. PMID 8322400. S2CID 3107793.
- Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. (June 2007). "Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group". Stroke. 38 (6): 2001–2023. doi:10.1161/strokeaha.107.183689. PMID 17478736.
- Hansen BM, Nilsson OG, Anderson H, Norrving B, Säveland H, Lindgren A (October 2013). "Long term (13 years) prognosis after primary intracerebral haemorrhage: a prospective population based study of long term mortality, prognostic factors and causes of death". Journal of Neurology, Neurosurgery, and Psychiatry. 84 (10): 1150–1155. doi:10.1136/jnnp-2013-305200. PMID 23715913. S2CID 40379279. Archived from the original on 2014-02-22.
External links
| Classification | D |
|---|
| Cerebrovascular diseases including stroke | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischaemic stroke |
| ||||||||||||
| Haemorrhagic stroke |
| ||||||||||||
| Aneurysm | |||||||||||||
| Other | |||||||||||||
| Neurotrauma | |
|---|---|
| Traumatic brain injury | |
| Spinal cord injury | |
| Peripheral nerves | |