| |
| |
| Names | |
|---|---|
| IUPAC name Mercury(I) sulfate | |
| Other names Mercurous sulfate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.029.084 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Hg2SO4 |
| Molar mass | 497.24 g/mol |
| Appearance | whitish-yellow crystals |
| Density | 7.56 g/cm |
| Solubility in water | 0.051 g/100 mL (25 °C) 0.09 g/100 mL (100 °C) |
| Solubility product (Ksp) | 6.5×10 |
| Solubility | soluble in dilute nitric acid, Insoluble in water, Soluble in hot sulfuric acid. |
| Magnetic susceptibility (χ) | −123.0·10 cm/mol |
| Structure | |
| Coordination geometry | monoclinic |
| Thermochemistry | |
| Heat capacity (C) | 132 J·mol·K |
| Std molar entropy (S298) |
200.7 J·mol·K |
| Std enthalpy of formation (ΔfH298) |
-743.1 kJ·mol |
| Related compounds | |
| Other anions | Mercury(I) fluoride Mercury(I) chloride Mercury(I) bromide Mercury(I) iodide |
| Other cations | Mercury(II) sulfate Cadmium sulfate Thallium(I) sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
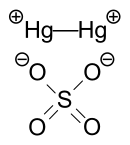
Mercury(I) sulfate, commonly called mercurous sulphate (UK) or mercurous sulfate (US) is the chemical compound Hg2SO4. Mercury(I) sulfate is a metallic compound that is a white, pale yellow or beige powder. It is a metallic salt of sulfuric acid formed by replacing both hydrogen atoms with mercury(I). It is highly toxic; it could be fatal if inhaled, ingested, or absorbed by skin.
Structure
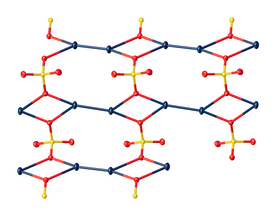
In the crystal, mercurous sulfate is made up of Hg2 center with an Hg-Hg distance of about 2.50 Å. The SO4 anions form both long and short Hg-O bonds ranging from 2.23 to 2.93 Å.
Focusing on the shorter Hg-O bonds, the Hg – Hg – O bond angle is 165°±1°.
Preparation
One way to prepare mercury(I) sulfate is to mix the acidic solution of mercury(I) nitrate with 1 to 6 sulfuric acid solution:,
- Hg2(NO3)2 + H2SO4 → Hg2SO4 + 2 HNO3
It can also be prepared by reacting an excess of mercury with concentrated sulfuric acid:
- 2 Hg + 2 H2SO4 → Hg2SO4 + 2 H2O + SO2
Use in electrochemical cells
Mercury(I) sulfate is often used in electrochemical cells. It was first introduced in electrochemical cells by Latimer Clark in 1872, It was then alternatively used in Weston cells made by George Augustus Hulett in 1911. It has been found to be a good electrode at high temperatures above 100 °C along with silver sulfate.
Mercury(I) sulfate has been found to decompose at high temperatures. The decomposition process is endothermic, and it occurs between 335 °C and 500 °C.
Mercury(I) sulfate has unique properties that make the standard cells possible. It has a rather low solubility (about one gram per liter); diffusion from the cathode system is not excessive; and it is sufficient to give a large potential at a mercury electrode.
References
- John Rumble (June 18, 2018). CRC Handbook of Chemistry and Physics (99 ed.). CRC Press. pp. 5–189. ISBN 978-1-138-56163-2.
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 5–19, ISBN 0-8493-0594-2
- Intermediate Inorganic Chemistry by J. W. Mellor, published by Longmans, Green and Company, London, 1941, page 388
- "Mercurous Sulfate | 7783-36-0".
- Matthias Weil; Michael Puchberger; Enrique J. Baran (2004). "Preparation and Characterization of Dimercury(I)Monofluorophosphate(V), Hg2PO3F: Crystal Structure, Thermal Behavior, Vibrational Spectra, and Solid-State P and F NMR Spectra". Inorg. Chem. 43 (26): 8330–8335. doi:10.1021/ic048741e. PMID 15606179.
- Dorm, E. (1969). "Structural Studies on Mercury(I) Compounds. VI. Crystal Structure of Mercury(I) Sulfate and Selenate". Acta Chemica Scandinavica. 23: 1607–15. doi:10.3891/acta.chem.scand.23-1607.
- Weil, Matthias (2014). "Crystal structure of Hg2SO4– a redetermination". Acta Crystallographica Section E. 70 (9): i44. doi:10.1107/S1600536814011155. PMC 4186147. PMID 25309168.
- ^ Google Books result, accessed 11 December 2010
- Mercurous Sulphate, cadmium sulphate, and the cadmium cell. by Hulett G. A. The physical review.1907. p.19.
- "Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide" by Mathieu Toupin, Thiery Brousse, and Daniel Belanger. Chem. Mater. 2002, 14, 3945–3952
- "Electromotive Force Studies of Cell, CdxHgy | CdSO4,(m) I Hg2SO4, Hg, in Dioxane-Water Media" by Somesh Chakrabarti and Sukumar Aditya. Journal of Chemical and Engineering Data, Vol.17, No. 1, 1972
- "Characterization of Lithium Sulfate as an Unsymmetrical-Valence Salt Bridge for the Minimization of Liquid Junction Potentials in Aqueous – Organic Solvent Mixtures" by Cristiana L. Faverio, Patrizia R. Mussini, and Torquato Mussini. Anal. Chem. 1998, 70, 2589–2595
- ^ "George Augustus Hulett: from Liquid Crystals to Standard Cell" by John T. Stock. Bull. Hist. Chem. Volume 25, Number 2, 2000, p.91-98
- Lietzke, M. H.; Stoughton, R. W. (November 1953). "The Behavior of the Silver—Silver Sulfate and the Mercury—Mercurous Sulfate Electrodes at High Temperatures 1". Journal of the American Chemical Society. 75 (21): 5226–5227. doi:10.1021/ja01117a024.(subscription required)
- "Sulphates of Mercury and Standard Cells." by Elliott, R. B. and Hulett, G. A. The Journal of Physical Chemistry 36.7 (1932): 2083–2086.
| Mercury compounds | |||
|---|---|---|---|
| Mercury(I) | |||
| Mercury(II) |
| ||
| Mercury(IV) |
| ||
| Amalgams | |||
| Mercury cations | |||