| This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (August 2024) |
| |
| Names | |
|---|---|
| Other names Mesyl azide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CH3N3O2S |
| Molar mass | 121.12 g·mol |
| Melting point | 18 °C (64 °F; 291 K) |
| Boiling point | 120 °C (248 °F; 393 K) decomposes |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
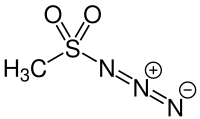
Methanesulfonyl azide is the azide of methanesulfonic acid. It is used as a reagent for the production of diazo compounds.
Preparation
Methanesulfonyl azide can be prepared from methanesulfonyl chloride by reaction with sodium azide in ethanol or methanol, Preparation in situ is also possible, for example in acetonitrile, and is advantageous to avoid explosion hazards.
Properties
Methanesulfonyl azide melts at 18 °C and decomposes from 120 °C. Like many other azides, it is explosive. At low temperature, methanesulfonyl azide crystallizes in the triclinic crystal system in the space group P1 with the lattice parameters a = 5.6240 Å; b = 5.9498 Å, c = 7.6329 Å, α = 72.216°, β = 70.897°, and γ = 88.601°, and two molecules per unit cell.
Reactions
Methanesulfonyl azide is a suitable reagent for introducing diazo compounds into other compounds. Historically, tosylazide was mainly used for this purpose. However, nowadays, compounds that are less explosive and/or form an amide after diazo transfer, which can be easily separated from the reaction products, are often used. In addition to imidazole-1-sulfonyl azide and 4-acetamidobenzenesulfonyl azide, methanesulfonyl azide is also utilized. The advantages of methanesulfonyl azide are particularly its simple and inexpensive production and the straightforward purification of the reaction mixture. Starting from Ω-bromoacetophenone, by reaction with trimethylphosphite, and then with sodium hydride and methanesulfonyl azide, a diazo reagent can be produced, which can convert aldehydes into alkynes. This method works similarly to the reaction with the Ohira-Bestmann reagent but is significantly more expensive to produce.
The photolysis of methanesulfonyl azide in a matrix of argon or neon yields a short-lived nitrene. If methanesulfonyl azide is irradiated in the presence of a hydrocarbon, methanesulfonyl amide is formed, as well as N-substituted derivatives by reaction with the hydrocarbon.
References
- ^ Deng, Guohai; Li, Dingqing; Wu, Zhuang; Li, Hongmin; Bernhardt, Eduard; Zeng, Xiaoqing (2016-07-21), "Methanesulfonyl Azide: Molecular Structure and Photolysis in Solid Noble Gas Matrices", The Journal of Physical Chemistry A, vol. 120, no. 28, pp. 5590–5597, doi:10.1021/acs.jpca.6b05533
- ^ Taber, Douglass F.; Ruckle, Robert E.; Hennessy, Michael J. (October 1986), "Mesyl azide: a superior reagent for diazo transfer", The Journal of Organic Chemistry, vol. 51, no. 21, pp. 4077–4078, doi:10.1021/jo00371a034
- ^ Lynch, Denis; O’Mahony, Rosella M.; McCarthy, Daniel G.; Bateman, Lorraine M.; Collins, Stuart G.; Maguire, Anita R. (2019-06-16), "Mechanistic Study of In Situ Generation and Use of Methanesulfonyl Azide as a Diazo Transfer Reagent with Real‐Time Monitoring by FlowNMR", European Journal of Organic Chemistry, vol. 2019, no. 22, pp. 3575–3580, doi:10.1002/ejoc.201900184, hdl:10468/8205
- Maas, Gerhard (2009-10-19), "New Syntheses of Diazo Compounds", Angewandte Chemie International Edition, vol. 48, no. 44, pp. 8186–8195, doi:10.1002/anie.200902785
- Taber, Douglass F.; Bai, Sha; Guo, Peng-fei (November 2008), "A convenient reagent for aldehyde to alkyne homologation", Tetrahedron Letters, vol. 49, no. 48, pp. 6904–6906, doi:10.1016/j.tetlet.2008.09.114, PMC 2634292, PMID 19946355
- Torimoto, Noboru; Shingaki, Tadao; Nagai, Toshikazu (February 1978), "Sensitized photolyses of methanesulfonyl azide in hydrocarbons", The Journal of Organic Chemistry, vol. 43, no. 4, pp. 631–633, doi:10.1021/jo00398a023