| |
| Names | |
|---|---|
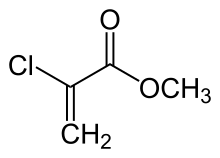
| Preferred IUPAC name Methyl 2-chloroprop-2-enoate | |
Other names
*2-Chloro-2-propenoic acid, methyl ester
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.001.181 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
SMILES
| |
| Properties | |
| Chemical formula | C4H5ClO2 |
| Molar mass | 120.53 g·mol |
| Appearance | Colorless liquid |
| Density | 1.189 g/cm at 68 °F (20 °C) |
| Boiling point | 52 °C; 126 °F; 325 K at 51.0 mmHg |
| Solubility in water | Insoluble in water |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Methyl 2-chloroacrylate is a colorless liquid used in manufacture of acrylic high polymer similar to polymethylmethacrylate. It is also used as a monomer for certain specialty polymers.
Methyl 2-chloroacrylate is polymerizable, insoluble in water, and a skin, eye, and lung irritant. Inhalation of vapors causes pulmonary edema. Trace amounts on the skin cause large blisters.
2-Aminothiazoline-4-carboxylic acid, an intermediate in the industrial synthesis of L-cysteine, is produced by the reaction of thiourea with methyl 2-chloroacrylate.
References
- ^ U.S. Environmental Protection Agency. 1998. Extremely Hazardous Substances (EHS) Chemical Profiles and Emergency First Aid Guides. Washington, D.C.: U.S. Government Printing Office
- "Cameo Chemicals". Retrieved 26 May 2012.
- Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker (2006). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_057.pub2. ISBN 978-3527306732.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)