| |
| Names | |
|---|---|
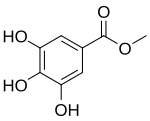
| Preferred IUPAC name Methyl 3,4,5-trihydroxybenzoate | |
| Other names
Methylgallate Gallic acid methyl ester Gallicin | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.492 |
| EC Number |
|
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H8O5 |
| Molar mass | 184.147 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Methyl gallate is a phenolic compound. It is the methyl ester of gallic acid.
Natural occurrences
It is found in Terminalia myriocarpa, Bergenia ciliata (hairy Bergenia) and Geranium niveum.
It is found in the fruit extract of Paeonia anomala.
It is also found in wine.
See also
References
- Marzouk, Mohamed S.A.; El-Toumy, Sayed A.A.; Moharram, Fatma A.; Shalaby, NM; Ahmed, AA (2002). "Pharmacologically Active Ellagitannins from Terminalia myriocarpa". Planta Medica. 68 (6): 523–7. doi:10.1055/s-2002-32549. PMID 12094296.
- Calzada, F; Cerda-García-Rojas, CM; Meckes, M; Cedillo-Rivera, R; Bye, R; Mata, R (1999). "Geranins a and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum". Journal of Natural Products. 62 (5): 705–9. doi:10.1021/np980467b. PMID 10346950.
- Oidovsambuu, S.; Kim, C.Y.; Kang, K.; Dulamjav, B.; Jigjidsuren, T.; Nho, C.W. (2013). "Protective effect of Paeonia anomala extracts and constituents against tert-butylhydroperoxide-induced oxidative stress in HepG2 cells". Planta Med. 79 (2): 116–122. doi:10.1055/s-0032-1328062. PMID 23349023. Retrieved 2016-04-20.
- Simultaneous Determination of Nonanthocyanin Phenolic Compounds in Red Wines by HPLC-DAD/ESI-MS. María Monagas, Rafael Suárez, Carmen Gómez-Cordovés and Begoña Bartolomé, Am J Enol Vitic. June 2005, 56, pages 139-147
| Phenolic acids (C6-C1) and their glycosides | |||||
|---|---|---|---|---|---|
| Monohydroxybenzoic acids |
| ||||
| Dihydroxybenzoic acids |
| ||||
| Trihydroxybenzoic acids |
| ||||
This article about an aromatic compound is a stub. You can help Misplaced Pages by expanding it. |