| |
| Names | |
|---|---|
| Preferred IUPAC name Methyl perchlorate | |
| Other names
Perchloric acid, methyl ester; | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
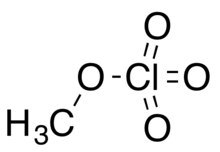
| Chemical formula | CH3ClO4 |
| Molar mass | 114.48 g·mol |
| Appearance | liquid |
| Boiling point | 52.0 °C (125.6 °F; 325.1 K) |
| Hazards | |
| Flash point | −14.8 ± 18.7 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Methyl perchlorate is an organic chemical compound with the chemical formula CH3ClO4. Its molecular structure is a methyl group covalently bonded by a single bond to a perchlorate group, CH3−O−Cl(=O)3, in which chlorine has an oxidation state of +7. Like many other perchlorates, it is a high energy material. It is also a toxic alkylating agent and exposure to the vapor can cause death. It can be prepared by treating iodomethane with a solution of silver perchlorate in benzene.
References
- Urben, Peter (2017). Bretherick's Handbook of Reactive Chemical Hazards. Elsevier. p. 116. ISBN 9780081010594.
- "Alkyl Perchlorate Esters - energeticchemical". Energeticchemical. Retrieved 13 June 2017.
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |