
| |
| Names | |
|---|---|
| IUPAC name N-pteridin-8(9H)-yl)benzoyl]-L-glutamic acid | |
| Other names
5,10-CH2-THF, MTHF | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | 5,10-methylenetetrahydrofolate |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H23N7O6 |
| Molar mass | 457.44 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
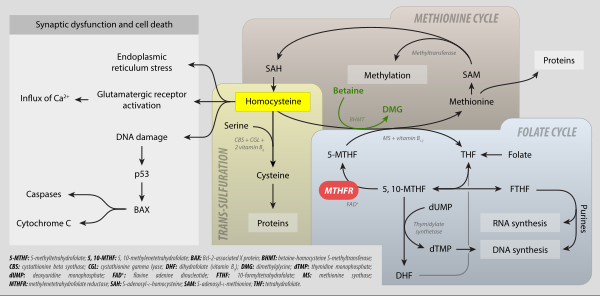
5,10-Methylenetetrahydrofolate (N5,N10-Methylenetetrahydrofolate; 5,10-CH2-THF) is cofactor in several biochemical reactions. It exists in nature as the diastereoisomer -5,10-methylene-THF.
As an intermediate in one-carbon metabolism, 5,10-CH2-THF converts to 5-methyltetrahydrofolate, 5-formyltetrahydrofolate, and methenyltetrahydrofolate. It is substrate for the enzyme methylenetetrahydrofolate reductase (MTHFR) It is mainly produced by the reaction of tetrahydrofolate with serine, catalyzed by the enzyme serine hydroxymethyltransferase.
Selected functions
Formaldehyde equivalent
Methylenetetrahydrofolate is a source of the equivalent of formaldehyde or CH2 in biosyntheses.
Methylenetetrahydrofolate is also an intermediate in the detoxification of formaldehyde.
Pyrimidine biosynthesis
It is the one-carbon donor for thymidylate synthase, for methylation of 2-deoxy-uridine-5-monophosphate (dUMP) to 2-deoxy-thymidine-5-monophosphate (dTMP). The coenzyme is necessary for the biosynthesis of thymidine and is the C1-donor in the reactions catalyzed by TS and thymidylate synthase (FAD).
Biomodulator
-5,10-methylene-THF is a biomodulator that has proven to enhance the desired cytotoxic antitumor effect of Fluorouracil (5-FU) and can bypass the metabolic pathway required by other folates (such as leucovorin) to achieve necessary activation. The active metabolite is being evaluated in clinical trials for patients with colorectal cancer in combination with 5-FU.
See also
References
- "Entrez Gene: MTHFR methylenetetrahydrofolate reductase (NAD(P)H)".
- Födinger M, Hörl WH, Sunder-Plassmann G (2000). "Molecular biology of 5,10-methylenetetrahydrofolate reductase". J Nephrol. 13 (1): 20–33. PMID 10720211.
- Marx, C. J.; Chistoserdova, L.; Lidstrom, M. E. (2003). "Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1". J. Bacteriol. 185 (24): 7160–8. doi:10.1128/jb.185.23.7160-7168.2003. PMC 296243. PMID 14645276.
- Danenberg, Peter V.; Gustavsson, Bengt; Johnston, Patrick; Lindberg, Per; Moser, Rudolf; Odin, Elisabeth; Peters, Godefridus J.; Petrelli, Nicholas (2016-10-01). "Folates as adjuvants to anticancer agents: Chemical rationale and mechanism of action". Critical Reviews in Oncology/Hematology. 106: 118–131. doi:10.1016/j.critrevonc.2016.08.001. ISSN 1879-0461. PMID 27637357.
| Enzyme cofactors | |||||||
|---|---|---|---|---|---|---|---|
| Active forms |
| ||||||
| Base forms | |||||||
| Vitamins (A11) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fat soluble |
| ||||||||
| Water soluble |
| ||||||||
| Combinations | |||||||||
| |||||||||