| |
| Names | |
|---|---|
| Preferred IUPAC name 1-Methylpyridin-1-ium | |
| Other names N-Methylpyridinium | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H8N |
| Molar mass | 94.134 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
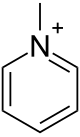
Methylpyridinium is an ion with the formula C5H5NCH+3. It is the N-methylated derivative of pyridine. It confers no color to its salts. The ion is classified as an quaternary ammonium ion.
Preparation and occurrence
Methylpyridinium is prepared by treating pyridine with dimethylsulfate:
- C5H5N + (CH3O)2SO2 → [C5H5NCH3]CH3OSO−3
It is found in some coffee products. It is not present in unroasted coffee beans, but is formed during roasting from its precursor chemical, trigonelline. It is under investigation by scientists regarding its potential anti-carcinogenic properties, particularly an effect on colon cancer.
Ionic liquid
The chloride salt of N-methylpyridinium behaves as an ionic liquid. Mixtures of that salt and zinc chloride have been characterised over the temperature range 150–200 °C (423–473 K).
See also
References
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2000). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. p. 558. doi:10.1002/14356007.a22_399. ISBN 9783527303854.
- E. A. Prill, S. M. McElvain (1935). "1-Methyl-2-Pyridone". Organic Syntheses. 15: 41. doi:10.15227/orgsyn.015.0041.
- ^ "Highly Active Compound Found In Coffee May Prevent Colon Cancer". ScienceDaily. Oct 15, 2003. Retrieved Oct 10, 2012.
- Boettler, U; Volz, N; Pahlke, G; Teller, N; Kotyczka, C; Somoza, V; Stiebitz, H; Bytof, G; et al. (2011). "Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo". Molecular Nutrition & Food Research. 55 (5): 798–802. doi:10.1002/mnfr.201100115. PMID 21448860.
- Simonis, L.; Coppe, C.; Glibert, J.; Claes, P. (1986). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – I. Phase diagram and heats of mixing". Thermochimica Acta. 99: 223–232. Bibcode:1986TcAc...99..223S. doi:10.1016/0040-6031(86)85285-6.
- Claes, P.; Simonis, L.; Glibert, J. (1986). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – II. Specific mass, electrical conductivity and viscosity". Electrochimica Acta. 31 (12): 1525–1530. doi:10.1016/0013-4686(86)87071-2.
- Claes, P. F.; Coppe, C. R.; Simonis, L. A.; Glibert, J. E. (1987). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – III. Solubility of hydrogen chloride under atmospheric pressure and comparison with zinc chloride—N-ethylpyridinium bromide mixtures". Journal of Chemical and Engineering Data. 32 (1): 70–72. doi:10.1021/je00047a020.
- Marković, R.; Minić, D. M. (1997). "Conductometric and thermal studies of fused Zn(II) salts containing methyl substituted pyridinium cations". Materials Chemistry and Physics. 50 (1): 20–24. doi:10.1016/S0254-0584(97)80178-2.
This coffee-related article is a stub. You can help Misplaced Pages by expanding it. |