| Reductive amination | |
|---|---|
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000335 |
Reductive amination (also known as reductive alkylation) is a form of amination that converts a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common method to make amines and is widely used in green chemistry since it can be done catalytically in one-pot under mild conditions. In biochemistry, dehydrogenase enzymes use reductive amination to produce the amino acid glutamate. Additionally, there is ongoing research on alternative synthesis mechanisms with various metal catalysts which allow the reaction to be less energy taxing, and require milder reaction conditions. Investigation into biocatalysts, such as imine reductases, have allowed for higher selectivity in the reduction of chiral amines which is an important factor in pharmaceutical synthesis.

Reaction process
Reductive amination occurs between a carbonyl such as an aldehyde or ketone and an amine in the presence of a reducing agent. The reaction conditions are neutral or weakly acidic.

Reaction Steps
- The nucelophilic amine reacts at the carbon of the carbonyl group to form a hemiaminal species
- reversible loss of one molecule of water from the hemiaminal species by alkylimino-de-oxo-bisubstitution to form the imine intermediate. The equilibrium between aldehyde/ketone and imine is shifted toward imine formation by dehydration.
- The intermediate imine can be isolated or reacted in-situ with a suitable reducing agent (e.g., sodium borohydride) to produce the amine product. Intramolecular reductive amination can also occur to afford a cyclic amine product if the amine and carbonyl are on the same molecule of starting material.
There are two ways to conduct a reductive amination reaction: direct and indirect.
Direct Reductive Amination
In a direct reaction, the carbonyl and amine starting materials and the reducing agent are combined and the reductions are done sequentially. These are often one-pot reactions since the imine intermediate is not isolated before the final reduction to the product. Instead, as the reaction proceeds, the imine becomes favoured for reduction over the carbonyl starting material. The two most common methods for direct reductive amination are hydrogenation with catalytic platinum, palladium, or nickel catalysts and the use of hydride reducing agents like cyanoborohydride (NaBH3CN).
Indirect Reductive Amination
Indirect reductive amination, also called a stepwise reduction, isolates the imine intermediate. In a separate step, the isolated imine intermediate is reduced to form the amine product.
Designing a reductive amination reaction
There are many considerations to be made when designing a reductive amination reaction.
- Chemoselectivity issues may arise since the carbonyl group can also be reduced.
- The reaction between the carbonyl and amine are in equilibrium, favouring the carbonyl unless water is removed from the system.
- reduction-sensitive intermediates may form in the reaction which can affect chemoselectivity.
- The amine substrate, imine intermediate, or amine product might deactivate the catalyst.
- Acyclic imines have E/Z isomers. This makes it difficult to create enantiopure chiral compounds through stereoselective reductions.
To solve the last issue, asymmetric reductive amination reactions can be used to synthesize an enantiopure product of chiral amines. In asymmetric reductive amination, a carbonyl that can be converted from achiral to chiral is used. The carbonyl undergoes condensation with an amine in the presence of H2 and a chiral catalyst to form the imine intermediate, which is then reduced to form the amine. However, this method is still limiting to synthesize primary amines which are non-selective and prone to overalkylation.
Common reducing agents
Palladium Hydride
Palladium hydride (H2/Pd) is a versatile reducing agent commonly used in reductive amination reactions. Its catalytic efficiency stems from the ability of palladium to adsorb hydrogen gas, forming active hydride species. These hydrides facilitate the reduction of imines or iminium ions—key intermediates in reductive amination—into secondary or tertiary amines. This reaction typically occurs under mild conditions with excellent selectivity, which often makes H2/Pd the first choice for synthesizing amines in pharmaceuticals and fine chemicals. Additionally, H2/Pd is compatible with a wide range of functional groups, further enhancing its utility in complex organic synthesis.
Sodium Borohydride
Sodium Borohydride (NaBH4) reduces both imines and carbonyl groups. However, it is not very selective and can reduce other reducible functional groups present in the reaction. To ensure that this does not occur, reagents with weak electrophilic carbonyl groups, poor nucleophilic amines and sterically hindered reactive centres should not be used, as these properties do not favour the reduction of the carbonyl to form an imine and increases the chance that other functional groups will be reduced instead.
Sodium Cyanoborohydride
Sodium cyanoborohydride (NaBH3CN) is soluble in hydroxylic solvents, stable in acidic solutions, and has different selectivities depending on the pH. At low pH values, it efficiently reduces aldehydes and ketones. As the pH increases, the reduction rate slows and instead, the imine intermediate becomes preferential for reduction. For this reason, NaBH3CN is an ideal reducing agent for one-pot direct reductive amination reactions that don't isolate the intermediate imine.
When used as a reducing agent, NaBH3CN can release toxic by-products like HCN and NaCN during work up.
Sodium Triacetoxyborohydride
Sodium triacetoxyborohydride (STAB, NaBH(OAc)3) is a common reducing agent for reductive aminations. STAB selectively reduces the imine intermediate formed through dehydration of the molecule. STAB is a weaker reductant than NaBH4, and can preferentially reduce the imine group in the presence of other reduction-sensitive functional groups. While STAB has also been reported as a selective reducing agent for aldehydes in the presence of keto groups, standard reductive amination reaction conditions greatly favour imine reduction to form an amine.
| H2/Pd | NaBH4 | NaBH(OAc) 3 | NaBH3CN | CO/Rh | |
| Selectivity | Low | Low | High | High | High |
| Atom economy | High | Solid wastes | Solid wastes | Solid wastes | High |
| Work up | Required | Not required | Not required | Not required | Required |
| Flammability | High | Low | High | High | High |
| Sensitivity to H2O, O2 | Low | High | High | High | Low |
| Toxicity | None | High, Carcinogen | Low | High | High |
Variations and related reactions
The reductive amination reaction is related to the Eschweiler–Clarke reaction, in which amines are methylated to tertiary amines, the Leuckart–Wallach reaction, and other amine alkylation methods such as the Mannich reaction and Petasis reaction.
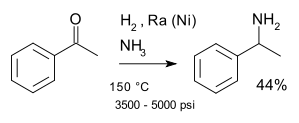
A classic named reaction is the Mignonac reaction (1921) involving reaction of a ketone with ammonia over a nickel catalyst. An example of this reaction is the synthesis of 1-phenylethylamine from acetophenone:
Additionally, many systems catalyze reductive aminations with hydrogenation catalysts. Generally, catalysis is preferred to stoichiometric reactions as they may improve reaction efficiency and atom economy, and produce less waste. These reactions can utilize homogeneous or heterogeneous catalyst systems. These systems provide alternative synthesis routes which are efficient, require fewer volatile reagents and are redox-economical. As well, this method can be used in the reduction of alcohols, along with aldehydes and ketones to form the amine product. One example of a heterogeneous catalytic system is the Ni-catalyzed reductive amination of alcohols. Nickel is commonly used as a catalyst for reductive amination because of its abundance and relatively good catalytic activity.

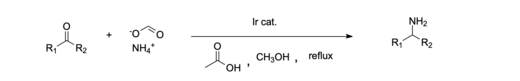
An example of a homogeneous catalytic system is the reductive amination of ketones done with an iridium catalyst. Homogenous Iridium (III) catalysts have been shown to be effective in the reductive amination of carboxylic acids, which in the past has been more difficult than aldehydes and ketones. Homogeneous catalysts are often favored because they are more environmentally and economically friendly compared to most heterogeneous systems.
In industry, tertiary amines such as triethylamine and diisopropylethylamine are formed directly from ketones with a gaseous mixture of ammonia and hydrogen and a suitable catalyst.
In green chemistry
Reductive amination is commonly used over other methods for introducing amines to alkyl substrates, such as SN2-type reactions with halides, since it can be done in mild conditions and has high selectivity for nitrogen-containing compounds. Reductive amination can occur sequentially in one-pot reactions, which eliminates the need for intermediate purifications and reduces waste. Some multistep synthetic pathways have been reduced to one step through one-pot reductive amination. This makes it a highly appealing method to produce amines in green chemistry.
Biochemistry
In biochemistry, dehydrogenase enzymes can catalyze the reductive amination of α-keto acids and ammonia to yield α-amino acids. Reductive amination is predominantly used for the synthesis of the amino acid glutamate starting from α-ketoglutarate, while biochemistry largely relies on transamination to introduce nitrogen in the other amino acids. The use of enzymes as a catalyst is advantageous because the enzyme active sites are often stereospecific and have the ability to selectively synthesize a certain enantiomer. This is useful in the pharmaceutical industry, particularly for drug-development, because enantiomer pairs can have different reactivities in the body. Additionally, enzyme biocatalysts are often quite selective in reactivity so they can be used in the presence of other functional groups, without the use of protecting groups. For instance a class of enzymes called imine reductases, IREDs, can be used to catalyze direct asymmetric reductive amination to form chiral amines.
In popular culture
In the critically acclaimed drama Breaking Bad, main character Walter White uses the reductive amination reaction to produce his high purity methamphetamine, relying on phenyl-2-propanone and methylamine.
See also
References
- ^ Thorpe, Thomas W.; Marshall, James R.; Harawa, Vanessa; Ruscoe, Rebecca E.; Cuetos, Anibal; Finnigan, James D.; Angelastro, Antonio; Heath, Rachel S.; Parmeggiani, Fabio; Charnock, Simon J.; Howard, Roger M.; Kumar, Rajesh; Daniels, David S. B.; Grogan, Gideon; Turner, Nicholas J. (7 April 2022). "Multifunctional biocatalyst for conjugate reduction and reductive amination". Nature. 604 (7904): 86–91. Bibcode:2022Natur.604...86T. doi:10.1038/s41586-022-04458-x. hdl:11311/1232494. ISSN 0028-0836. PMID 35388195. S2CID 248001189.
- ^ Abdel-Magid, Ahmed F.; Carson, Kenneth G.; Harris, Bruce D.; Maryanoff, Cynthia A.; Shah, Rekha D. (1 January 1996). "Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures 1". The Journal of Organic Chemistry. 61 (11): 3849–3862. doi:10.1021/jo960057x. ISSN 0022-3263. PMID 11667239.
- ^ Tripathi, Rama P.; Verma, Shyam S.; Pandey, Jyoti; Tiwari, Vinod K. (2008). "Recent Development on Catalytic Reductive Amination and Applications". Current Organic Chemistry. 12 (13): 1093–1115. doi:10.2174/138527208785740283.
- Sawant, Rajiv T.; Waghmode, Suresh B. (13 March 2010). "Intramolecular reductive amination strategy to the synthesis of (R)-N-Boc-2-hydroxymethylmorpholine, N-(3,4-dichlorobenzyl)(R)-2-hydroxymethylmorpholine, and (R)-2-benzylmorpholine". Tetrahedron. 66 (11): 2010–2014. doi:10.1016/j.tet.2010.01.047. ISSN 0040-4020.
- ^ Wang, Chao; Xiao, Jianliang (2013), Li, Wei; Zhang, Xumu (eds.), "Asymmetric Reductive Amination", Stereoselective Formation of Amines, vol. 343, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 261–282, doi:10.1007/128_2013_484, ISBN 978-3-642-53928-2, PMID 24158548, retrieved 6 November 2023
- ^ Reshi, Noor U Din; Saptal, Vitthal B.; Beller, Matthias; Bera, Jitendra K. (19 November 2021). "Recent Progress in Transition-Metal-Catalyzed Asymmetric Reductive Amination". ACS Catalysis. 11 (22): 13809–13837. doi:10.1021/acscatal.1c04208. ISSN 2155-5435. S2CID 240250685.
- ^ Borch, Richard F.; Durst, H. Dupont (July 1969). "Lithium cyanohydridoborate, a versatile new reagent". Journal of the American Chemical Society. 91 (14): 3996–3997. Bibcode:1969JAChS..91.3996B. doi:10.1021/ja01042a078. ISSN 0002-7863.
- Abdel-Magid, Ahmed F.; Carson, Kenneth G.; Harris, Bruce D.; Maryanoff, Cynthia A.; Shah, Rekha D. (1 January 1996). "Reductive Amination of Aldehydes and Ketones with Sodium Triacetoxyborohydride. Studies on Direct and Indirect Reductive Amination Procedures1". The Journal of Organic Chemistry. 61 (11): 3849–3862. doi:10.1021/jo960057x. ISSN 0022-3263.
- Abdel-Magid, Ahmed F.; Mehrman, Steven J. (1 September 2006). "A Review on the Use of Sodium Triacetoxyborohydride in the Reductive Amination of Ketones and Aldehydes". Organic Process Research & Development. 10 (5): 971–1031. doi:10.1021/op0601013. ISSN 1083-6160.
- Oliphant, Shannon J.; Morris, Robert H. (30 August 2022). "Density Functional Theory Study on the Selective Reductive Amination of Aldehydes and Ketones over Their Reductions to Alcohols Using Sodium Triacetoxyborohydride". ACS Omega. 7 (34): 30554–30564. doi:10.1021/acsomega.2c04056. PMC 9434773. PMID 36061668.
- Podyacheva, Evgeniya; Afanasyev, Oleg I.; Tsygankov, Alexey A.; Makarova, Maria; Chusov, Denis (July 2019). "Hitchhiker's Guide to Reductive Amination". Synthesis. 51 (13): 2667–2677. doi:10.1055/s-0037-1611788. ISSN 0039-7881.
- George, Frederick & Saunders, Bernard (1960). Practical Organic Chemistry, 4th Ed. London: Longman. p. 223. ISBN 9780582444072.
- Mignonac, Georges (1921). "Nouvelle méthode générale de préparation des amines à partir des aldéhydes ou des cétones" [New general method for preparation of amines from aldehydes or ketones]. Comptes rendus (in French). 172: 223.
- Robinson, John C.; Snyder, H. R. (1955). "α-Phenylethylamine". Organic Syntheses. doi:10.1002/0471264180.os023.27; Collected Volumes, vol. 3, p. 717.
- ^ Huang, Hao; Wei, Yuejun; Cheng, Yuran; Xiao, Shuwen; Chen, Mingchih; Wei, Zuojun (7 October 2023). "The Acquisition of Primary Amines from Alcohols through Reductive Amination over Heterogeneous Catalysts". Catalysts. 13 (10): 1350. doi:10.3390/catal13101350. ISSN 2073-4344.
- ^ Ouyang, Lu; Miao, Rui; Yang, Zhanhui; Luo, Renshi (1 February 2023). "Iridium-catalyzed reductive amination of carboxylic acids". Journal of Catalysis. 418: 283–289. doi:10.1016/j.jcat.2023.01.030. ISSN 0021-9517.
- Burns, Noah Z.; Baran, Phil S.; Hoffmann, Reinhard W. (6 April 2009). "Redox Economy in Organic Synthesis". Angewandte Chemie International Edition. 48 (16): 2854–2867. doi:10.1002/anie.200806086. ISSN 1433-7851. PMID 19294720.
- Kalbasi, Roozbeh Javad; Mazaheri, Omid (2015). "Synthesis and characterization of hierarchical ZSM-5 zeolite containing Ni nanoparticles for one-pot reductive amination of aldehydes with nitroarenes". Catalysis Communications. 69: 86–91. doi:10.1016/j.catcom.2015.05.016.
- Chernyshev, Victor M.; Ananikov, Valentine P. (21 January 2022). "Nickel and Palladium Catalysis: Stronger Demand than Ever". ACS Catalysis. 12 (2): 1180–1200. doi:10.1021/acscatal.1c04705. ISSN 2155-5435. S2CID 245795966.
- Tanaka, Kouichi; Miki, Takashi; Murata, Kunihiko; Yamaguchi, Ayumi; Kayaki, Yoshihito; Kuwata, Shigeki; Ikariya, Takao; Watanabe, Masahito (6 September 2019). "Reductive Amination of Ketonic Compounds Catalyzed by Cp*Ir(III) Complexes Bearing a Picolinamidato Ligand". The Journal of Organic Chemistry. 84 (17): 10962–10977. doi:10.1021/acs.joc.9b01565. ISSN 0022-3263. PMID 31362498. S2CID 199000460.
- ^ Van Praet, Sofie; Preegel, Gert; Rammal, Fatima; Sels, Bert F. (12 May 2022). "One-Pot Consecutive Reductive Amination Synthesis of Pharmaceuticals: From Biobased Glycolaldehyde to Hydroxychloroquine". ACS Sustainable Chemistry & Engineering. 10 (20): 6503–6508. doi:10.1021/acssuschemeng.2c00570. ISSN 2168-0485. S2CID 248767494.
- He, Jian; Chen, Lulu; Liu, Shima; Song, Ke; Yang, Song; Riisager, Anders (2020). "Sustainable access to renewable N-containing chemicals from reductive amination of biomass-derived platform compounds". Green Chemistry. 22 (20): 6714–6747. doi:10.1039/d0gc01869d. ISSN 1463-9262. S2CID 225001665.
- Metzler, D. E. "Biochemistry—The Chemical Reactions of Living Cells, Vol. 2" 2nd Ed. Academic Press: San Diego, 2003.
- ^ Wohlgemuth, Roland; Littlechild, Jennifer (22 July 2022). "Complexity reduction and opportunities in the design, integration and intensification of biocatalytic processes for metabolite synthesis". Frontiers in Bioengineering and Biotechnology. 10. doi:10.3389/fbioe.2022.958606. hdl:10871/130495. ISSN 2296-4185.
- Brooks, W. H.; Guida, W. C.; Daniel, K. G. (2011). "The Significance of Chirality in Drug Design and Development". Current Topics in Medicinal Chemistry. 11 (7): 760–770. doi:10.2174/156802611795165098. PMC 5765859. PMID 21291399.
- ^ Wu, Kai; Huang, Junhai; Shao, Lei (22 November 2022). "Imine Reductases: Multifunctional Biocatalysts with Varying Active Sites and Catalytic Mechanisms". ChemCatChem. 14 (22). doi:10.1002/cctc.202200921. ISSN 1867-3880. S2CID 252271457.
External links
- Current methods for reductive amination
- Industrial reductive amination at BASF