
| |
| |
| Names | |
|---|---|
| IUPAC name Magnesium hydroxide | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.013.792 |
| EC Number |
|
| E number | E528 (acidity regulators, ...) |
| Gmelin Reference | 485572 |
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | Mg(OH)2 |
| Molar mass | 58.3197 g/mol |
| Appearance | White solid |
| Odor | Odorless |
| Density | 2.3446 g/cm |
| Melting point | 350 °C (662 °F; 623 K) decomposes |
| Solubility in water |
|
| Solubility product (Ksp) | 5.61×10 |
| Magnetic susceptibility (χ) | −22.1×10 cm/mol |
| Refractive index (nD) | 1.559 |
| Structure | |
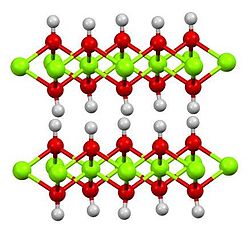
| Crystal structure | Hexagonal, hP3 |
| Space group | P3m1 No. 164 |
| Lattice constant | a = 0.312 nm, c = 0.473 nm |
| Thermochemistry | |
| Heat capacity (C) | 77.03 J/mol·K |
| Std molar entropy (S298) |
64 J·mol·K |
| Std enthalpy of formation (ΔfH298) |
−924.7 kJ·mol |
| Gibbs free energy (ΔfG) | −833.7 kJ/mol |
| Pharmacology | |
| ATC code | A02AA04 (WHO) G04BX01 (WHO) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P261, P280, P304+P340, P305+P351+P338, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 8500 mg/kg (rat, oral) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
| Other anions | Magnesium oxide |
| Other cations | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low solubility in water (Ksp = 5.61×10). Magnesium hydroxide is a common component of antacids, such as milk of magnesia.
Preparation
Treating the solution of different soluble magnesium salts with alkaline water induces the precipitation of the solid hydroxide Mg(OH)2:
- Mg + 2 OH → Mg(OH)2
As Mg
is the second most abundant cation present in seawater after Na
, it can be economically extracted directly from seawater by alkalinisation as described here above. On an industrial scale, Mg(OH)2 is produced by treating seawater with lime (Ca(OH)2). A volume of 600 m (160,000 US gal) of seawater gives about 1 tonne (2,200 lb) of Mg(OH)2. Ca(OH)2 (Ksp = 5.02×10) is far more soluble than Mg(OH)2 (Ksp = 5.61×10) and drastically increases the pH value of seawater from 8.2 to 12.5. The less soluble Mg(OH)
2 precipitates because of the common ion effect due to the OH
added by the dissolution of Ca(OH)
2:
- Mg + Ca(OH)2 → Mg(OH)2 + Ca
For seawater brines, precipitating agents other than Ca(OH)2 can be utilized, each with their own nuances:
- Use of Ca(OH)2 can yield CaSO4 or CaCO3, which reduces the final purity of Mg(OH)2.
- NH4OH, can produce explosive nitrogen trichloride when the brine is used for chlorine production.
- NaOH as the precipitating agent has longer settling times and is difficult to filter.
It has been demonstrated that sodium hydroxide, NaOH, is the better precipitating agent compared to Ca(OH)2 and NH4OH due to higher recovery and purity rates, and the settling and filtration time can be improved at low temperatures and higher concentration of precipitates. Methods involving the use of precipitating agents are typically batch processes.
It is also possible to obtain Mg(OH)2 from seawater using electrolysis chambers separated with a cation exchange membrane. This process is continuous, lower-cost, and produces oxygen gas, hydrogen gas, sulfuric acid (if Na2SO4 is used; NaCl can alternatively be used to yield HCl), and Mg(OH)2 of 98% or higher purity. It is crucial to deaerate the seawater to mitigate co-precipitation of calcium precipitates.
Uses
Precursor to MgO
Most Mg(OH)2 that is produced industrially, as well as the small amount that is mined, is converted to fused magnesia (MgO). Magnesia is valuable because it is both a poor electrical conductor and an excellent thermal conductor.
Medical
Only a small amount of the magnesium from magnesium hydroxide is usually absorbed by the intestine (unless one is deficient in magnesium). However, magnesium is mainly excreted by the kidneys; so long-term, daily consumption of milk of magnesia by someone suffering from kidney failure could lead in theory to hypermagnesemia. Unabsorbed magnesium is excreted in feces; absorbed magnesium is rapidly excreted in urine.

Applications
Antacid
As an antacid, magnesium hydroxide is dosed at approximately 0.5–1.5 g in adults and works by simple neutralization, in which the hydroxide ions from the Mg(OH)2 combine with acidic H ions (or hydronium ions) produced in the form of hydrochloric acid by parietal cells in the stomach, to produce water.
Laxative
As a laxative, magnesium hydroxide is dosed at 5–10 grams (0.18–0.35 oz), and works in a number of ways. First, Mg is poorly absorbed from the intestinal tract, so it draws water from the surrounding tissue by osmosis. Not only does this increase in water content soften the feces, it also increases the volume of feces in the intestine (intraluminal volume) which naturally stimulates intestinal motility. Furthermore, Mg ions cause the release of cholecystokinin (CCK), which results in intraluminal accumulation of water and electrolytes, and increased intestinal motility. Some sources claim that the hydroxide ions themselves do not play a significant role in the laxative effects of milk of magnesia, as alkaline solutions (i.e., solutions of hydroxide ions) are not strongly laxative, and non-alkaline Mg solutions, like MgSO4, are equally strong laxatives, mole for mole.
History of milk of magnesia
On May 4, 1818, American inventor Koen Burrows received a patent (No. X2952) for magnesium hydroxide. In 1829, Sir James Murray used a "condensed solution of fluid magnesia" preparation of his own design to treat the Lord Lieutenant of Ireland, the Marquess of Anglesey, for stomach pain. This was so successful (advertised in Australia and approved by the Royal College of Surgeons in 1838) that he was appointed resident physician to Anglesey and two subsequent Lords Lieutenant, and knighted. His fluid magnesia product was patented two years after his death, in 1873.
The term milk of magnesia was first used by Charles Henry Phillips in 1872 for a suspension of magnesium hydroxide formulated at about 8% w/v. It was sold under the brand name Phillips' Milk of Magnesia for medicinal usage.
USPTO registrations show that the terms "Milk of Magnesia" and "Phillips' Milk of Magnesia" have both been assigned to Bayer since 1995. In the UK, the non-brand (generic) name of "Milk of Magnesia" and "Phillips' Milk of Magnesia" is "Cream of Magnesia" (Magnesium Hydroxide Mixture, BP).
As food additive
It is added directly to human food, and is affirmed as generally recognized as safe by the FDA. It is known as E number E528.
Magnesium hydroxide is marketed for medical use as chewable tablets, as capsules, powder, and as liquid suspensions, sometimes flavored. These products are sold as antacids to neutralize stomach acid and relieve indigestion and heartburn. It also is a laxative to alleviate constipation. As a laxative, the osmotic force of the magnesia acts to draw fluids from the body. High doses can lead to diarrhea, and can deplete the body's supply of potassium, sometimes leading to muscle cramps.
Some magnesium hydroxide products sold for antacid use (such as Maalox) are formulated to minimize unwanted laxative effects through the inclusion of aluminum hydroxide, which inhibits the contractions of smooth muscle cells in the gastrointestinal tract, thereby counterbalancing the contractions induced by the osmotic effects of the magnesium hydroxide.
Other niche uses
Magnesium hydroxide is also a component of antiperspirant.
Waste water treatment
Magnesium hydroxide powder is used industrially to neutralize acidic wastewaters. It is also a component of the Biorock method of building artificial reefs. The main advantage of Mg(OH)
2 over Ca(OH)
2, is to impose a lower pH better compatible with that of seawater and sea life: pH 10.5 for Mg(OH)
2 in place of pH 12.5 with Ca(OH)
2.
Fire retardant
Natural magnesium hydroxide (brucite) is used commercially as a fire retardant. Most industrially used magnesium hydroxide is produced synthetically. Like aluminum hydroxide, solid magnesium hydroxide has smoke suppressing and flame retardant properties. This property is attributable to the endothermic decomposition it undergoes at 332 °C (630 °F):
- Mg(OH)2 → MgO + H2O
The heat absorbed by the reaction retards the fire by delaying ignition of the associated substance. The water released dilutes combustible gases. Common uses of magnesium hydroxide as a flame retardant include additives to cable insulation, insulation plastics, roofing, and various flame retardant coatings.
Mineralogy

Brucite, the mineral form of Mg(OH)2 commonly found in nature also occurs in the 1:2:1 clay minerals amongst others, in chlorite, in which it occupies the interlayer position normally filled by monovalent and divalent cations such as Na, K, Mg and Ca. As a consequence, chlorite interlayers are cemented by brucite and cannot swell nor shrink.
Brucite, in which some of the Mg cations have been substituted by Al cations, becomes positively charged and constitutes the main basis of layered double hydroxide (LDH). LDH minerals as hydrotalcite are powerful anion sorbents but are relatively rare in nature.
Brucite may also crystallize in cement and concrete in contact with seawater. Indeed, the Mg cation is the second-most-abundant cation in seawater, just behind Na and before Ca. Because brucite is a swelling mineral, it causes a local volumetric expansion responsible for tensile stress in concrete. This leads to the formation of cracks and fissures in concrete, accelerating its degradation in seawater.
For the same reason, dolomite cannot be used as construction aggregate for making concrete. The reaction of magnesium carbonate with the free alkali hydroxides present in the cement porewater also leads to the formation of expansive brucite.
- MgCO3 + 2 NaOH → Mg(OH)2 + Na2CO3
This reaction, one of the two main alkali–aggregate reaction (AAR) is also known as alkali–carbonate reaction.
See also
- Portlandite – calcium hydroxyde: Ca(OH)
2
References
- Patnaik, Pradyot (2003). Handbook of inorganic chemicals. New York: McGraw-Hill. ISBN 0-07-049439-8. OCLC 50252041.
- Toshiaki Enoki and Ikuji Tsujikawa (1975). "Magnetic Behaviours of a Random Magnet, NipMg(1−p)(OH)2". J. Phys. Soc. Jpn. 39 (2): 317–323. Bibcode:1975JPSJ...39..317E. doi:10.1143/JPSJ.39.317.
- ^ Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
- ^ "Magnesium Hydroxide". American Elements. Retrieved May 9, 2019.
- Handbook of Chemistry and Physics (76th ed.). CRC Press. 12 March 1996. ISBN 0849305969.
- Rumble, John (June 18, 2018). CRC Handbook of Chemistry and Physics (99th ed.). CRC Press. pp. 5–188. ISBN 978-1138561632.
- ^ Seeger, Margarete; Otto, Walter; Flick, Wilhelm; Bickelhaupt, Friedrich; Akkerman, Otto S. "Magnesium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_595.pub2. ISBN 978-3527306732.
- Fontana, Danilo; Forte, Federica; Pietrantonio, Massimiliana; Pucciarmati, Stefano; Marcoaldi, Caterina (2023-12-01). "Magnesium recovery from seawater desalination brines: a technical review". Environment, Development and Sustainability. 25 (12): 13733–13754. Bibcode:2023EDSus..2513733F. doi:10.1007/s10668-022-02663-2.
- Sano, Yoshihiko; Hao, YiJia; Kuwahara, Fujio (2018-11-01). "Development of an electrolysis based system to continuously recover magnesium from seawater". Heliyon. 4 (11): e00923. Bibcode:2018Heliy...400923S. doi:10.1016/j.heliyon.2018.e00923. PMC 6249789. PMID 30839823.
- "magnesium hydroxide". Global Library of Women's Medicine. Archived from the original on 14 January 2018. Retrieved 2023-03-14.
- Tedesco, Frances J.; DiPiro, Joseph T. (1985). "Laxative use in constipation". The American Journal of Gastroenterology. 80 (4): 303–309. PMID 2984923.
- Patent USX2952 - Magnesia, medicated, liquid - Google Patents
- Michael Hordern. A World Elsewhere (1993), p. 2.
- "Sir James Murray's condensed solution of fluid magnesia". The Sydney Morning Herald. Vol. 21, no. 2928. October 7, 1846. p. 1, column 4.
- Ulster History. Sir James Murray – Inventor of Milk of Magnesia. 1788 to 1871 Archived 2011-06-05 at the Wayback Machine, 24 February 2005
- When was Phillips' Milk of Magnesia introduced? Archived 2017-06-22 at the Wayback Machine FAQ, phillipsrelief.com, accessed 4 July 2016
- results from the TARR web server: Milk of Magnesia
- results from the TARR web server: Phillips' Milk of Magnesia
- "Compound Summary for CID 14791 - Magnesium Hydroxide". PubChem.
- Magnesium Hydroxide – Revolution Health
- Washington, Neena (2 August 1991). Antacids and Anti Reflux Agents. Boca Raton, FL: CRC Press. p. 10. ISBN 0-8493-5444-7.
- Milk of Magnesia Makes Good Antiperspirant
- Aileen Gibson and Michael Maniocha. White Paper: The Use Of Magnesium Hydroxide Slurry For Biological Treatment Of Municipal and Industrial Wastewater, August 12, 2004
- Rothon, RN (2003). Particulate Filled Polymer Composites. Shrewsbury, UK: Rapra Technology. pp. 53–100.
- Hollingbery, LA; Hull, TR (2010). "The Thermal Decomposition of Huntite and Hydromagnesite - A Review". Thermochimica Acta. 509 (1–2): 1–11. doi:10.1016/j.tca.2010.06.012.
- Hollingbery, LA; Hull, TR (2010). "The Fire Retardant Behaviour of Huntite and Hydromagnesite - A Review". Polymer Degradation and Stability. 95 (12): 2213–2225. doi:10.1016/j.polymdegradstab.2010.08.019.
- Hollingbery, LA; Hull, TR (2012). "The Fire Retardant Effects of Huntite in Natural Mixtures with Hydromagnesite". Polymer Degradation and Stability. 97 (4): 504–512. doi:10.1016/j.polymdegradstab.2012.01.024.
- Hollingbery, LA; Hull, TR (2012). "The Thermal Decomposition of Natural Mixtures of Huntite and Hydromagnesite". Thermochimica Acta. 528: 45–52. Bibcode:2012TcAc..528...45H. doi:10.1016/j.tca.2011.11.002.
- Hull, TR; Witkowski, A; Hollingbery, LA (2011). "Fire Retardant Action of Mineral Fillers". Polymer Degradation and Stability. 96 (8): 1462–1469. doi:10.1016/j.polymdegradstab.2011.05.006. S2CID 96208830.
| Magnesium compounds | |
|---|---|
| Hydroxides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Urologicals, including antispasmodics (G04B) | |
|---|---|
| Acidifiers | |
| Urinary antispasmodics (primarily antimuscarinics) | |
| Other urologicals |
|
| Drugs for acid related disorders: Antacids (A02A) | |
|---|---|
| Magnesium (increases motility) | |
| Aluminium (decreases motility) | |
| Calcium | |
| Sodium | |
| Combinations and complexes of aluminium, calcium and magnesium | |
| Drugs for constipation (laxatives and cathartics) (A06) | |
|---|---|
| Stool softeners | |
| Stimulant laxatives | |
| Bulk-forming laxatives | |
| Lubricant laxatives | |
| Osmotic laxatives | |
| Enemas | |
| Opioid antagonists | |
| Others | |