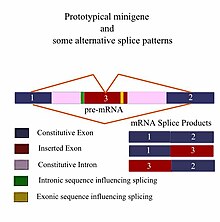
A minigene is a minimal gene fragment that includes an exon and the control regions necessary for the gene to express itself in the same way as a wild type gene fragment. This is a minigene in its most basic sense. More complex minigenes can be constructed containing multiple exons and intron(s). Minigenes provide a valuable tool for researchers evaluating splicing patterns both in vivo and in vitro biochemically assessed experiments. Specifically, minigenes are used as splice reporter vectors (also called exon-trapping vectors) and act as a probe to determine which factors are important in splicing outcomes. They can be constructed to test the way both cis-regulatory elements (RNA effects) and trans-regulatory elements (associated proteins/splicing factors) affect gene expression.
History
Minigenes were first described as the somatic assembly of DNA segments and consisted of DNA regions known to encode the protein and the flanking regions required to express the protein. The term was first used in a paper in 1977 to describe the cloning of two minigenes that were designed to express a peptide.
RNA splicing was discovered in the late 1970s through the study of adenoviruses that invade mammals and replicate inside them. Researchers identified RNA molecules that contained sequences from noncontiguous parts of the virus’s genome. This discovery led to the conclusion that regulatory mechanisms existed which affected mature RNA and the genes it expresses. Using minigenes as a splice reporting vector to explore the effects of RNA splicing regulation naturally followed and remains the major use of minigenes to date.
Types
In order to provide a good minigene model, the gene fragment should have all of the necessary elements to ensure it exhibits the same alternative splicing (AS) patterns as the wild type gene, i.e., the length of the fragment must include all upstream and downstream sequences which can affect its splicing. Therefore, most minigene designs begin with a thorough in silico analysis of the requirements of the experiment before any "wet" lab work is conducted. With the advent of Bioinformatics and widespread use of computers, several good programs now exist for the identification of cis-acting control regions that affect the splicing outcomes of a gene and advanced programs can even consider splicing outcomes in various tissue types. Differences in minigenes are usually reflected in the final size of the fragment, which is in turn a reflection of the complexity of the minigene itself. The number of foreign DNA elements (exon and introns) inserted into the constitutive exons and introns of a given fragment varies with the type of experiment and the information being sought. A typical experiment might involve wild type minigenes which are expected to express genes normally in a comparison run against genetically engineered allelic variations which replace the wild-type gene and have been cloned into the same flanking sequences as the original fragment. These types of experiments help to determine the effect of various mutations on pre-mRNA splicing.
Construction
Once a suitable genomic fragment is chosen (Step 1), the exons and introns of the fragment can be inserted and amplified, along with the flanking constitutive exons and introns of the original gene, by PCR. The primers for PCR can be chosen so that they leave "sticky ends" at 3' sense and anti-sense strands (Step 2). These "sticky-ends" can be easily incorporated into a TOPO Vector by ligation into a commercially available source which has ligase already attached to it at the sight of incorporation (Step 3). The subsequent TOPO Vectors can be transfected into E.coli cells (Step 4). After incubation, total RNA can be extracted from the bacterial colonies and analyzed using RT-PCR to quantify ratios of exon inclusion/exclusion (step 5). The minigene can be transfected into different cell types with various splicing factors to test trans-acting elements (Step 6). The expressed genes or the proteins they encode can be analyzed to evaluate splicing components and their effects via a variety of methods including hybridization or size-exclusion chromatography.

Uses
RNA splicing errors have been estimated to occur in a third of genetic diseases. To understand pathogenesis and identify potential targets of therapeutic intervention in these diseases, explicating the splicing elements involved is essential. Determining the complete set of components involved in splicing presents many challenges due to the abundance of alternative splicing, which occurs in most human genes, and the specificity in which splicing is carried out in vivo. Splicing is distinctly conducted from cell type to cell type and across different stages of cellular development. Therefore, it is critical that any in vitro or bioinformatic assumptions about splicing regulation are confirmed in vivo. Minigenes are used to elucidate cis-regulatory elements, trans-regulatory elements and other regulators of pre-mature RNA splicing in vivo. Minigenes have been applied to the study of a diverse array of genetic diseases due to the aforementioned abundance of alternatively spliced genes and the specificity and variation observed in splicing regulation. The following are examples of minigene use in various diseases. While it is not an exhaustive list, it does provide a better understanding of how minigenes are utilized.
Endocrine diseases
RNA splicing errors can have drastic effects on how proteins function, including the hormones secreted by the endocrine system. These effects on hormones have been identified as the cause of many endocrine disorders including thyroid-related pathological conditions, rickets, hyperinsulinemic hypoglycemia and congenital adrenal hyperplasia. One specific example of a splicing error causing an endocrine disease that has been studied using minigenes is a type of growth hormone deficiency called isolated growth hormone deficiency (IGHD), a disease that results in growth failure. IGHD type II is an autosomal dominant form caused by a mutation in the intervening sequence (IVS) adjacent to exon 3 of the gene encoding growth hormone 1, the GH-1 gene. This mutated form of IVS3 causes exon 3 to be skipped in the mRNA product. The mRNA (-E3) encodes a truncated form of hGH that then inhibits normal hGH secretion. Minigenes were used to determine that a point mutation within an intron splice enhancer (ISE) embedded in IVS3 was to blame for the skipping of E3. Moreover, it was determined that the function of the ISE is influenced by a nearby transposable AC element, revealing that this particular splicing error is caused by a trans-acting factor.
Neurodegenerative diseases
Accumulation of tau protein is associated with neurodegenerative diseases including Alzheimer's and Parkinson's diseases as well as other tauopathies. Tau protein isoforms are created by alternative splicing of exons 2, 3 and 10. The regulation of tau splicing is specific to stage of development, physiology and location. Errors in tau splicing can occur in both exons and introns and, depending on the error, result in changes to protein structure or loss of function. Aggregation of these abnormal tau proteins correlates directly with pathogenesis and disease progression. Minigenes have been used by several researchers to help understand the regulatory components responsible for mRNA splicing of the TAU gene.
Cancer
Cancer is a complex, heterogeneous disease that can be hereditary or the result of environmental stimuli. Minigenes are used to help oncologists understand the roles pre-mRNA splicing plays in different cancer types. Of particular interest are cancer specific genetic mutations that disrupt normal splicing events, including those affecting spliceosome components and RNA-binding proteins such as heterogeneous nuclear ribonucleoparticules (hnRNP), serine/arginine-rich (SR) proteins and small ribonucleoproteins (snRNP). Proteins encoded by aberrantly spliced pre-mRNAs are functionally different and contribute to the characteristic anomalies exhibited by cancer cells, including their ability to proliferate, invade and undergo angiogenesis, and metastasis. Minigenes help researchers identify genetic mutations in cancer that result in splicing errors and determine the downstream effects those splicing errors have on gene expression. Using knowledge obtained from studies employing minigenes, oncologists have proposed tests designed to detect products of abnormal gene expression for diagnostic purposes. Additionally, the prospect of using minigenes as a cancer immunotherapy is being explored.
See also
References
- ^ Stoss, O; Stoilov, P; Hartmann, AM; Nayler, O; Stamm, S (Dec 1999). "The in vivo minigene approach to analyze tissue-specific splicing". Brain Research. Brain Research Protocols. 4 (3): 383–94. doi:10.1016/s1385-299x(99)00043-4. PMID 10592349.
- ^ Cooper, Thomas A. (December 2005). "Use of minigene systems to dissect alternative splicing elements". Methods. 37 (4): 331–340. doi:10.1016/j.ymeth.2005.07.015. PMID 16314262.
- ^ Desviat, LR; Pérez, B; Ugarte, M (2012). "Minigenes to Confirm Exon Skipping Mutations". Exon Skipping. Methods Mol. Biol. Vol. 867. pp. 37–47. doi:10.1007/978-1-61779-767-5_3. ISBN 978-1-61779-766-8. PMID 22454053.
- Poonian, MS; McComas, WW; Nussbaum, AL (1977). "Chemical synthesis of two deoxyribododecanucleotides for the attachment of restriction termini to an artificial minigene". Gene. 1 (5–6): 357–72. doi:10.1016/0378-1119(77)90040-3. PMID 590743.
- Clancy, S (2008). "RNA splicing: introns, exons and spliceosome". Nature Education. 1 (31).
- Burge, Christopher. "Burge Lab Software". Archived from the original on 30 March 2019. Retrieved 7 May 2014.
- Divina, Petr; Kvitkovicova, Andrea; Buratti, Emanuele; Vorechovsky, Igor (14 January 2009). "Ab initio prediction of mutation-induced cryptic splice-site activation and exon skipping". European Journal of Human Genetics. 17 (6): 759–765. doi:10.1038/ejhg.2008.257. PMC 2947103. PMID 19142208.
- Grodecká, Lucie; Lockerová, Pavla; Ravčuková, Barbora; Buratti, Emanuele; Baralle, Francisco E.; Dušek, Ladislav; Freiberger, Tomáš; Spilianakis, Charalampos Babis (21 February 2014). "Exon First Nucleotide Mutations in Splicing: Evaluation of In Silico Prediction Tools". PLOS ONE. 9 (2): e89570. Bibcode:2014PLoSO...989570G. doi:10.1371/journal.pone.0089570. PMC 3931810. PMID 24586880.
- Barash, Yoseph; Vaquero-Garcia, Jorge; González-Vallinas, Juan; Xiong, Hui; Gao, Weijun; Lee, Leo J.; Frey, Brendan J. (2013). "AVISPA: a web tool for the prediction and analysis of alternative splicing". Genome Biology. 14 (10): R114. doi:10.1186/gb-2013-14-10-r114. PMC 4014802. PMID 24156756.
- "Steps in producing a TOPO Vector". Life sciences. Retrieved 7 May 2014.
- Lim, Kian; Huat; Ferraris, Luciana; Filloux, Madeleine E.; Raphael, Benjamin J.; Fairbrother, William G. (2011). "Using positional distribution to identify splicing elements and predict pre-mRNA processing defects in human genes". Proceedings of the National Academy of Sciences. 108 (27): 11093–6. Bibcode:2011PNAS..10811093H. doi:10.1073/pnas.1101135108. PMC 3131313. PMID 21685335.
- ^ Stamm, Stefan. "Stamms-lab.net". Archived from the original on 9 December 2013. Retrieved 26 March 2014.
- Rosaria de Miranda, Elizabete (2009). "Splicing variants impact in thyroid normal physiology and pathological conditions". Arq Bras Endocrinol Metabol. 53 (6): 709–714. doi:10.1590/S0004-27302009000600003. PMID 19893912.
- Mullis, PE (2010). "Genetics of isolated growth hormone deficiency". J Clin Res Pediatr Endocrinol. 2 (2): 52–62. doi:10.4274/jcrpe.v2i2.52. PMC 3014602. PMID 21274339.
- ^ Kar, Amar; Fushimi, Kazuo; Zhou, Xiaohong; Ray, Payal; Shi, Chen; Chen, Xiaoping; Liu, Zhiren; Chen, She; Wu, Jane Y. (2011). "RNA Helicase p68 (DDX5) Regulates tau Exon 10 Splicing by Modulating a Stem-Loop Structure at the 5′ Splice Site". Mol. Cell. Biol. 31 (9): 1812–1821. doi:10.1128/MCB.01149-10. PMC 3133221. PMID 21343338.
- ^ Rodriguez-Martin, Teresa; Karen Anthony; Mariano A. Garcia-Blanco; S. Gary Mansfield; Brian H. Anderton; Jean-Marc Gallo (2009). "Correction of tau mis-splicing caused by FTDP-17 MAPT mutations by spliceosome-mediated RNA trans-splicing". Hum Mol Genet. 18 (17): 3266–3273. doi:10.1093/hmg/ddp264. PMC 2722988. PMID 19498037.
- Anfossi, M; Vuono, R; Maletta, R; Virdee, K; Mirabelli, M; Colao, R; Puccio, G; Bernardi, L; Frangipane, F; Gallo, M; Geracitano, S; Tomaino, C; Curcio, SA; Zannino, G; Lamenza, F; Duyckaerts, C; Spillantini, MG; Losso, MA; Bruni, AC (2011). "Compound heterozygosity of 2 novel MAPT mutations in frontotemporal dementia". Neurobiol Aging. 32 (4): 757.e1–757.e11. doi:10.1016/j.neurobiolaging.2010.12.013. PMID 21295377. S2CID 5176440.
- Rajan, P.; Elliott, DJ; Robson, CN; Leung, HY (Aug 2009). "Alternative splicing and biological heterogeneity in prostate cancer". Nat Rev Urol. 6 (8): 454–460. doi:10.1038/nrurol.2009.125. PMID 19657379. S2CID 30664940.
- Adler, AS; McCleland, ML; Yee, S; Yaylaoglu, M; Hussain, S; Cosino, E; Quinones, G; Modrusan, Z; Seshagiri, S; Torres, E; Chopra, VS; Haley, B; Zhang, Z; Blackwood, EM; Singh, M; Junttila, M; Stephan, JP; Liu, J; Pau, G; Fearon, ER; Jiang, Z; Firestein, R (May 2014). "An integrative analysis of colon cancer identifies an essential function for PRPF6 in tumor growth". Genes Dev. 28 (10): 1068–84. doi:10.1101/gad.237206.113. PMC 4035536. PMID 24788092.
- ^ Guo, Rong; Yong Li; Jinying Ning; Dan Sun; Lianjun Lin; Xinmin Liu (2013). "HnRNP A1/A2 and SF2/ASF Regulate Alternative Splicing of Interferon Regulatory Factor-3 and Affect Immunomodulatory Functions in Human Non-Small Cell Lung Cancer Cells". PLOS ONE. 8 (4): e62729. Bibcode:2013PLoSO...862729G. doi:10.1371/journal.pone.0062729. PMC 3639176. PMID 23658645.
- Acedo, Alberto; David J Sanz; Mercedes Durán; Mar Infante; Lucía Pérez-Cabornero; Cristina Miner; Eladio A Velasco (2012). "Comprehensive splicing functional analysis of DNA variants of the BRCA2 gene by hybrid minigenes". Breast Cancer Research. 14 (3): R87. doi:10.1186/bcr3202. PMC 3446350. PMID 22632462.
- Di Giacomo, D.; Gaildrat, P; Abuli, A; Abdat, J; Frébourg, T; Tosi, M; Martins, A (2013). "Functional analysis of a large set of BRCA2 exon 7 variants highlights the predictive value of hexamer scores in detecting alterations of exonic splicing regulatory elements". Hum. Mutat. 34 (11): 1547–57. doi:10.1002/humu.22428. PMID 23983145. S2CID 22874730.
- Daniotti, Jose L.; Aldo A. Vilcaes; Vanina Torres Demichelis; Fernando M. Ruggiero; Macarena Rodriguez-Walker (2013). "Glycosylation of Glycolipids in Cancer: Basis for Development of Novel Therapeutic Approaches". Front Oncol. 3: 306. doi:10.3389/fonc.2013.00306. PMC 3867695. PMID 24392350.
- Aurisicchio, L; Fridman, A; Bagchi, A; Scarselli, E; La Monica, N; Ciliberto, G (Jan 2014). "A novel minigene scaffold for therapeutic cancer vaccines". Oncoimmunology. 3 (1): e27529. doi:10.4161/onci.27529. PMC 4002591. PMID 24790791.
Further reading
- "Alternative pre-mRNA Splicing: Theory and Protocols", by Stefan Stamm, Chris Smith and Reinhard Lührmann ISBN 978-3527326068
- "Molecular Diagnostics, Second edition", by Ed. by George P. Patrinos and Whilhelm Ansorge ISBN 0123745373
- "DNA Vaccines" edited by Hildegun Ertl ISBN 1461349257
- "Alternative Splicing and Disease (Progress in Molecular and Subcellular Biology)" by Philippe Jeanteur ISBN 3540344489
External links
- Stefan Stamm's web page at the University of Kentucky. Good overview of minigene research.
- Christopher Burge's Lab at M.I.T. website Archived 2019-03-30 at the Wayback Machine. A Good site for theoretical analysis of splicing.
- UCSC Genome Browser. Large database for retrieving information on genes.