| This article is missing information about potential applications. Please expand the article to include this information. Further details may exist on the talk page. (October 2014) |
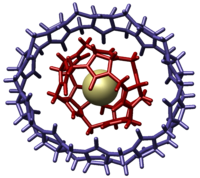
Molecular gyroscopes are chemical compounds or supramolecular complexes containing a rotor that moves freely relative to a stator, and therefore act as gyroscopes. Though any single bond or triple bond permits a chemical group to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a crystal structure with low packing density or by physically surrounding the rotor avoiding steric contact. A qualitative distinction can be made based on whether the activation energy needed to overcome rotational barriers is higher than the available thermal energy. If the activation energy required is higher than the available thermal energy, the rotor undergoes "site exchange", jumping in discrete steps between local energy minima on the potential energy surface. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotor can behave more like a macroscopic freely rotating inertial mass.

For example, several studies in 2002 with a p-phenylene rotor found that some structures using variable-temperature (VT) solid-state C CPMAS and quadrupolar echo H NMR were able to detect a two-site exchange rate of 1.6 MHz (over 10/second at 65 °C), described as "remarkably fast for a phenylene group in a crystalline solid", with steric barriers of 12–14 kcal/mol. However, tert-butyl modification of the rotor increased the exchange rate to over 10 per second at room temperature, and the rate for inertially rotating p-phenylene without barriers is estimated to be approximately 2.4 x 10 revolutions per second.
| Year of publication | Rotor | Stator | Linkage | Reference |
|---|---|---|---|---|
| 2002 | cucurbituril | cucurbituril | noncovalent | |
| 2007 | p-phenylene | two m-methoxy-substituted trityl groups | triple bonds | |
| 2007 | p-phenylene | triply bridged trityl cage | triple bonds | |
| 2010 | halogen-substituted p-phenylene | silaalkane chains | single bonds | |
| 2014 | p-phenylene | trityl groups bridged by photoactive azobenzene bridge | triple bonds | |
| 2015 | H–Pt–H | two tri-tert-butylphosphine groups | Pt–P bonds |
References
- ^ Day, Anthony I.; Blanch, Rodney J.; Arnold, Alan P.; Lorenzo, Susan; Lewis, Gareth R.; Dance, Ian (2002). "A Cucurbituril-Based Gyroscane: A New Supramolecular Form". Angew. Chem. Int. Ed. 41 (2): 275–277. doi:10.1002/1521-3773(20020118)41:2<275::AID-ANIE275>3.0.CO;2-M.
- ^ Tinh-Alfredo V. Khuong; Hung Dang; Peter D. Jarowski; Emily F. Maverick & Miguel A. Garcia-Garibay (2007). "Rotational Dynamics in a Crystalline Molecular Gyroscope by Variable-Temperature C NMR, H NMR, X-Ray Diffraction, and Force Field Calculations" (PDF). J. Am. Chem. Soc. 129 (4): 839–845. doi:10.1021/ja064325c. Archived from the original (PDF) on 2014-10-06. Retrieved 2014-10-04.
- ^ Wataru Setaka; Soichiro Ohmizu; Mitsuo Kira (2010). "Molecular Gyroscope Having a Halogen-substituted p-Phenylene Rotator and Silaalkane Chain Stators". Chemistry Letters. 39 (5): 468–469. doi:10.1246/cl.2010.468.
- Jose E. Nuez; Arunkumar Natarajan; Saeed I. Khan & Miguel A. Garcia-Garibay (2007). "Synthesis of a Triply-Bridged Molecular Gyroscope by a Directed Meridional Cyclization Strategy" (PDF). Org. Lett. 9 (18): 3559–3561. doi:10.1021/ol071379y. Archived from the original (PDF) on 2014-10-06. Retrieved 2014-10-04.
- Patrick Commins & Miguel A. Garcia-Garibay (2014). "Photochromic Molecular Gyroscope with Solid State Rotational States Determined by an Azobenzene Bridge". J. Org. Chem. 79 (4): 1611–1619. doi:10.1021/jo402516n.
- Ernest Prack; Christopher A. O’Keefe; Jeremy K. Moore; Angel Lai; Alan J. Lough; Peter M. Macdonald; Mark S. Conradi; Robert W. Schurko; Ulrich Fekl (2015). "A Molecular Rotor Possessing an H–M–H "Spoke" on a P–M–P "Axle": A Platinum(II) trans-Dihydride Spins Rapidly Even at 75 K". J. Am. Chem. Soc. 137 (42): 13464–13467. doi:10.1021/jacs.5b08213.