| |
| Names | |
|---|---|
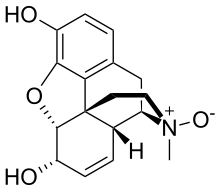
| IUPAC name (4R,4aR,7S,7aR,12bS)-3-Methyl-2,3,4,4a,7,7a-hexahydro-1H-4,12-methanobenzofuroisoquinoline-7,9-diol 3-oxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.010.324 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C17H19NO4 |
| Molar mass | 301.342 g·mol |
| Legal status |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Morphine-N-oxide (genomorphine) is an active opioid metabolite of morphine. Morphine itself, in trials with rats, is 11–22 times more potent than morphine-N-oxide subcutaneously and 39–89 times more potent intraperitoneally. However, pretreatment with amiphenazole or tacrine increases the potency of morphine-N-oxide in relation to morphine (intraperitoneally more so than in subcutaneous administration). A possible explanation is that morphine-N-oxide is rapidly inactivated in the liver and impairment of inactivation processes or enzymes increases functionality.
Morphine-N-oxide can also form as a decomposition product of morphine outside the body and may show up in assays of opium and poppy straw concentrate. Codeine and the semi-synthetics such as heroin, dihydrocodeine, dihydromorphine, hydromorphone, and hydrocodone also have equivalent amine oxide derivatives.
Morphine-N-oxide has a DEA ACSCN of 9307 and annual production quota of 655 grams in 2013. It is a Schedule I controlled substance in the US.
See also
References
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- Fennessy, M. R. (1968). "The analgesic action of morphine-n-oxide". British Journal of Pharmacology. 34 (2): 337–344. doi:10.1111/j.1476-5381.1968.tb07055.x. PMC 1703337. PMID 5687589.
- 21 U.S.C. § 812(b)(1) United States Code via Cornell University's Legal Information Institute. Retrieved on 2024-10-01.
This analgesic-related article is a stub. You can help Misplaced Pages by expanding it. |