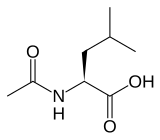
 (S)-(−)-N-Acetyl-leucine | |
| Names | |
|---|---|
| IUPAC name 2-Acetamido-4-methylpentanoic acid | |
| Other names N-Acetylleucine; N-Acetyl-L-Leucine | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| 3DMet |
|
| Beilstein Reference | 1724849 (S)-(−) |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| EC Number |
|
| Gmelin Reference | 985259 (S)-(−) |
| KEGG |
|
| MeSH | acetylleucine |
| PubChem CID | |
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H15NO3 |
| Molar mass | 173.212 g·mol |
| Appearance | White crystals |
| Melting point | −115 to −113 °C; −175 to −172 °F; 158 to 160 K |
| log P | −0.265 |
| Acidity (pKa) | 3.666 |
| Basicity (pKb) | 10.331 |
| Pharmacology | |
| ATC code | N07CA04 (WHO) |
| Related compounds | |
| Related compounds | ENU |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Acetylleucine (N-acetyl-leucine) is a modified leucine amino acid used in the treatment of vertigo and cerebellar ataxia.
Two forms exist: acetyl-DL-leucine (sold under the brand Tanganil, among others) and N-acetyl-L-leucine (levacetylleucine).
Acetylleucine is also being developed as a possible treatment for several neurological disorders by IntraBio Inc. Clinical trials with acetylleucine for the treatment of three orphan, fatal, neurodegenerative disorders are underway: Niemann-Pick disease type C, GM2 gangliosidoses (Tay-Sachs and Sandhoff diseases), and ataxia–telangiectasia. In 2020, IntraBio announced the successful multinational clinical trial results of the Niemann-Pick type C clinical trial. IntraBio is also investigating acetylleucine for the treatment of common inherited and acquired neurological diseases including Lewy body dementia, amyotrophic lateral sclerosis, restless legs syndrome, multiple sclerosis, and migraine Acetylleucine has received orphan drug designations from the U.S. Food & Drug Administration (FDA) and the European Commission.
References
- "N-Acetyl-DL-leucine". PubChem Open Chemistry Database. Retrieved 26 March 2017.
- "N07CA04 (acetylleucine)". WHO Collaborating Centre for Drug Statistics Methodology. Norwegian Institute of Public Health. 19 December 2016. Retrieved 26 March 2017.
- Oertel WH, Janzen A, Henrich MT, Geibl FF, Sittig E, Meles SK, et al. (2 September 2024). "Acetyl-DL-leucine in two individuals with REM sleep behavior disorder improves symptoms, reverses loss of striatal dopamine-transporter binding and stabilizes pathological metabolic brain pattern—case reports". Nature Communications. 15 (1): 7619. doi:10.1038/s41467-024-51502-7. ISSN 2041-1723. PMC 11369233. PMID 39223119.
- "IntraBio". Archived from the original on 1 August 2019. Retrieved 1 August 2019.
- "N-Acetyl-L-Leucine for Niemann-Pick Disease, Type C (NPC) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 1 August 2019.
- "N-Acetyl-L-Leucine for GM2 Gangliosdisosis (Tay-Sachs and Sandhoff Disease) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 1 August 2019.
- "N-Acetyl-L-Leucine for Ataxia-Telangiectasia (A-T) - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 1 August 2019.
- "IntraBio Reports Further Detail on Positive Data from IB1001 Multinational Clinical Trial for the Treatment of Niemann-Pick disease Type C". intrabio.com. 19 October 2020. Retrieved 1 August 2021.
- Passmore P (15 April 2014). "A clinical trial to test amlodipine as a new treatment for vascular dementia". doi:10.1186/isrctn31208535.
{{cite journal}}: Cite journal requires|journal=(help) - Strupp M, Bayer O, Feil K, Straube A (1 February 2019). "Prophylactic treatment of migraine with and without aura with acetyl-dl-leucine: a case series". Journal of Neurology. 266 (2): 525–529. doi:10.1007/s00415-018-9155-6. ISSN 1432-1459. PMID 30547273. S2CID 56148131.
- "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- "Search Orphan Drug Designations and Approvals". www.accessdata.fda.gov. Retrieved 3 August 2019.
- "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
- "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
- "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
- "Public Health - European Commission". Union Register of medicinal products. Retrieved 3 August 2019.
| Drugs used for vertigo (N07C) | |
|---|---|