| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Butan-1-amine | |
Other names
| |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | NBA |
| Beilstein Reference | 605269 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.364 |
| EC Number |
|
| Gmelin Reference | 1784 |
| MeSH | n-butylamine |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1125 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C4H11N |
| Molar mass | 73.139 g·mol |
| Appearance | Colorless liquid |
| Odor | fishy, ammoniacal |
| Density | 740 mg ml |
| Melting point | −49 °C; −56 °F; 224 K |
| Boiling point | 77 to 79 °C; 170 to 174 °F; 350 to 352 K |
| Solubility in water | Miscible |
| log P | 1.056 |
| Vapor pressure | 9.1 kPa (at 20 °C) |
| Henry's law constant (kH) |
570 μmol Pa kg |
| Basicity (pKb) | 3.22 |
| Magnetic susceptibility (χ) | -58.9·10 cm/mol |
| Refractive index (nD) | 1.401 |
| Viscosity | 500 µPa s (at 20 °C) |
| Thermochemistry | |
| Heat capacity (C) | 188 J K mol |
| Std enthalpy of formation (ΔfH298) |
−128.9–−126.5 kJ mol |
| Std enthalpy of combustion (ΔcH298) |
−3.0196–−3.0174 MJ mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H225, H302, H312, H314, H332 |
| Precautionary statements | P210, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) |
 |
| Flash point | −7 °C (19 °F; 266 K) |
| Autoignition temperature |
312 °C (594 °F; 585 K) |
| Explosive limits | 1.7–9.8% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
|
| LCLo (lowest published) | 4000 ppm (rat, 4 hr) 263 ppm (mouse, 2 hr) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | C 5 ppm (15 mg/m) |
| REL (Recommended) | C 5 ppm (15 mg/m) |
| IDLH (Immediate danger) | 300 ppm |
| Safety data sheet (SDS) | hazard.com |
| Related compounds | |
| Related alkanamines | |
| Related compounds | 2-Methyl-2-nitrosopropane |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
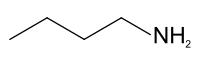
n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Its vapours are heavier than air and it produces toxic oxides of nitrogen during combustion.
Synthesis and reactions
It is produced by the reaction of ammonia and alcohols over alumina:
- CH3(CH2)3OH + NH3 → CH3(CH2)3NH2 + H2O
n-Butylamine is a weak base. The pKa of is 10.78.
n-Butylamine exhibits reactions typical of other simple alkyl amines, i.e., alkylation, acylation, condensation with carbonyls. It forms complexes with metal ions, examples being cis- and trans-.
Uses

This compound is used as an ingredient in the manufacture of pesticides (such as thiocarbazides), pharmaceuticals, and emulsifiers. It is also a precursor for the manufacture of N,N′-dibutylthiourea, a rubber vulcanization accelerator, and n-butylbenzenesulfonamide, a plasticizer of nylon. It is used in the synthesis of fengabine, the fungicide benomyl, and butamoxane, and the antidiabetic tolbutamide.
Safety
The LD50 to rats through the oral exposure route is 366 mg/kg.
In regards to occupational exposures to n-butylamine, the Occupational Safety and Health Administration and National Institute for Occupational Safety and Health have set occupational exposure limits at a ceiling of 5 ppm (15 mg/m) for dermal exposure.
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0079". National Institute for Occupational Safety and Health (NIOSH).
- ^ "N-Butylamine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- PubChem. "Butylamine". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-02-15.
- H. K. Hall, Jr. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- Rochon, Fernande D.; Buculei, Viorel (2004). "Multinuclear NMR Study and Crystal Structures of Complexes of the Types cis- and trans-Pt(amine)2I2". Inorganica Chimica Acta. 357 (8): 2218–2230. doi:10.1016/j.ica.2003.10.039.
- Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke, "Amines, Aliphatic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a02_001
- "n-Butylamine MSDS" (PDF). Archived from the original (PDF) on 2013-11-12. Retrieved 2013-11-12.
- CDC - NIOSH Pocket Guide to Chemical Hazards
External links
 Media related to N-Butylamine at Wikimedia Commons
Media related to N-Butylamine at Wikimedia Commons