| |
| Identifiers | |
|---|---|
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C40H46ClNO9 |
| Molar mass | 720.26 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
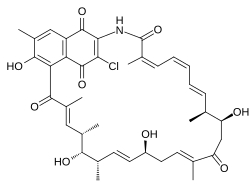
Naphthomycin A is a type of naphthomycin. It was isolated as a yellow pigment from Streptomyces collinus and it shows antibacterial, antifungal, and antitumor activities. Naphthomycins have the longest ansa aliphatic carbon chain of the ansamycin family. Biosynthetic origins of the carbon skeleton from PKS1 were investigated by feeding 13C-labeled precursors and subsequent 13C-NMR product analysis. Naphthomycin gene clusters have been cloned and sequenced to confirm involvement in biosynthesis via deletion of a 7.2kb region. Thirty-two genes were identified in the 106kb cluster.
References
- Kang, Q.; Y. Shenb; L. Bai (2012). "Biosynthesis of 3,5-AHBA-derived natural products". Nat. Prod. Rep. 29 (2): 243–263. doi:10.1039/c2np00019a. PMID 22193711.
- Balerna, M.; W. Keller-Schierlein; C. Martius; H. Zahner (1969). "Stoffwechselprodukte von Mikroorganismen". Arch. Microbiol. 65 (4): 303–317. doi:10.1007/BF00412210. PMID 4988744. S2CID 31145406.
- Bai, L.; Y. Wu; Q. Kang; Y. Shen; Z. Deng (2012). Patent. CN: 2012-10300294: 225.
{{cite journal}}: Missing or empty|title=(help) - August, P.R.; L. Tang; Y.J. Yoon; S. Ning; R. MuEller; T.W. Yu; M. Taylor; D. Hoffman; C.G. Kim; X. Zahng; C.R. Hutchinson; H.G. Floss (1998). "Biosynthesis of the ansamycin antibiotic rifamycin: Deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699". Chem. Biol. 5 (2): 69–79. doi:10.1016/S1074-5521(98)90141-7. PMID 9512878.
- Kang, Q; Y. Shenb; L. Bai (2012). "Biosynthesis of 3,5-AHBA-derived natural products". Nat. Prod. Rep. 29 (2): 243–263. doi:10.1039/c2np00019a. PMID 22193711.
This systemic antibiotic-related article is a stub. You can help Misplaced Pages by expanding it. |